ACID AND BASE REACTIONS IN WATER Chapter 8

ACID AND BASE REACTIONS IN WATER Chapter 8
KEY KNOWLEDGE • 8 A - The Brønsted-Lowry theory of acids and bases including polyprotic acids and amphiprotic species, and writing of balanced ionic equations for their reactions with water including states • 8 A - The distinction between strong and weak acids and bases, and between concentrated and dilute acids and bases, including common examples • 8 C- The reactions of acids with metals, carbonates and hydroxides including balanced full and ionic equations, with states indicated

INTRODUCING ACIDS AND BASES Chapter 8 A
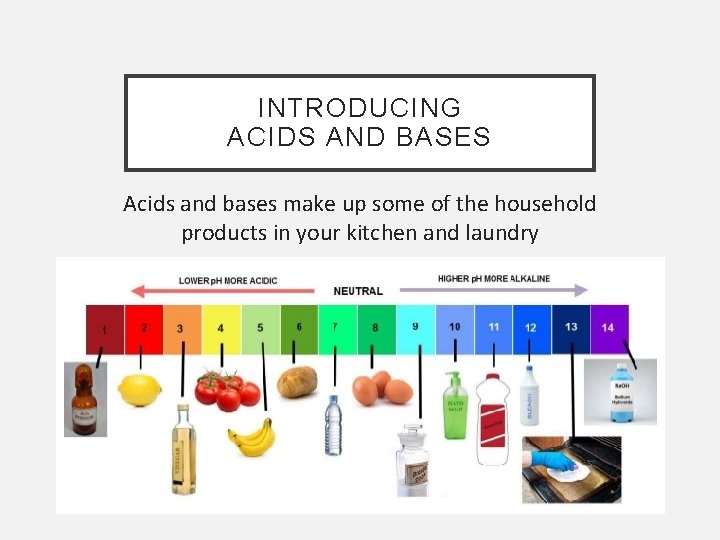
INTRODUCING ACIDS AND BASES Acids and bases make up some of the household products in your kitchen and laundry
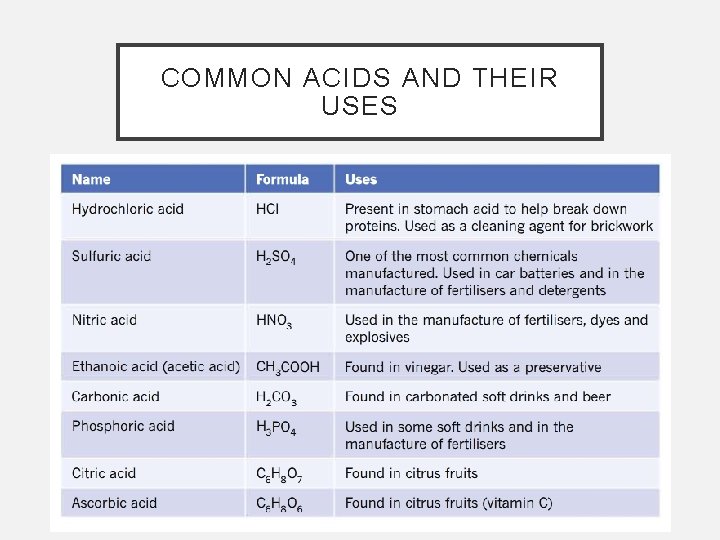
COMMON ACIDS AND THEIR USES

COMMON BASES AND THEIR USES • Bases are effective cleaners because they react with fats and oils to produce water-soluble soaps • A soluble base is referred to as an alkali
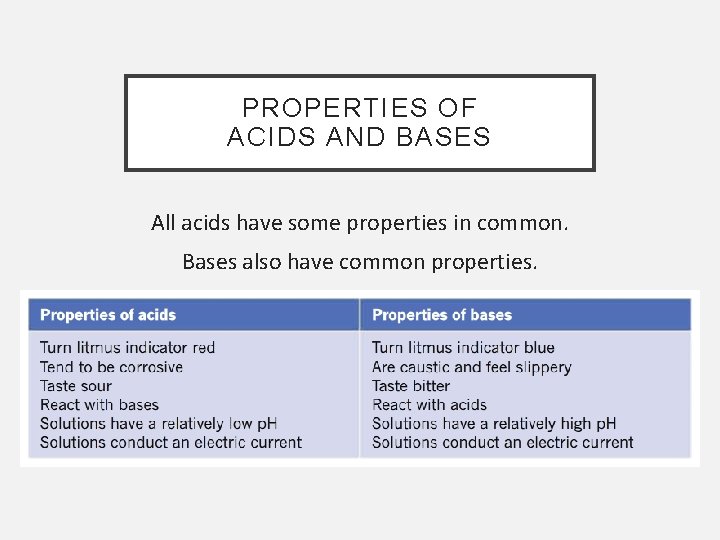
PROPERTIES OF ACIDS AND BASES All acids have some properties in common. Bases also have common properties.
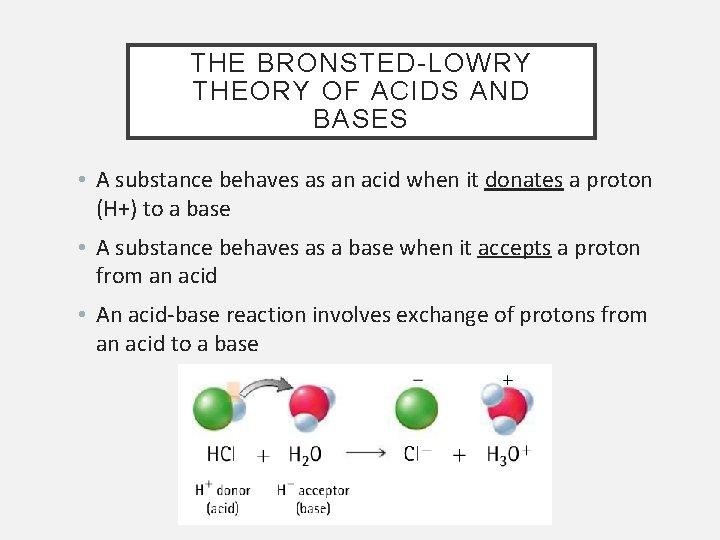
THE BRONSTED-LOWRY THEORY OF ACIDS AND BASES • A substance behaves as an acid when it donates a proton (H+) to a base • A substance behaves as a base when it accepts a proton from an acid • An acid-base reaction involves exchange of protons from an acid to a base
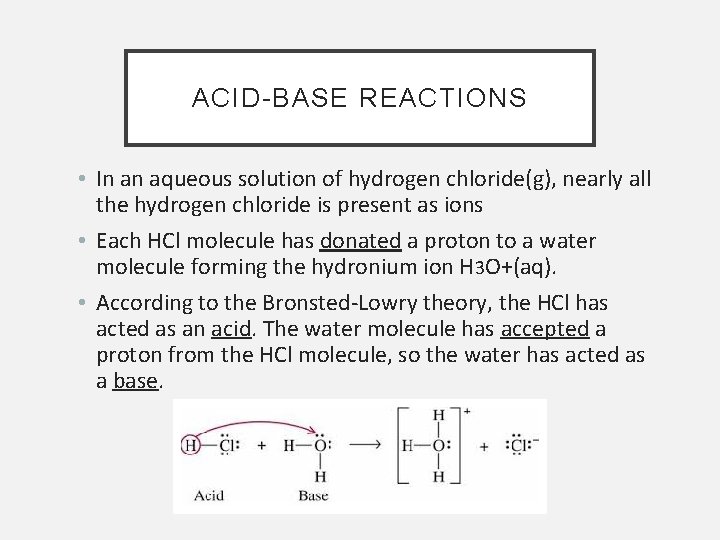
ACID-BASE REACTIONS • In an aqueous solution of hydrogen chloride(g), nearly all the hydrogen chloride is present as ions • Each HCl molecule has donated a proton to a water molecule forming the hydronium ion H 3 O+(aq). • According to the Bronsted-Lowry theory, the HCl has acted as an acid. The water molecule has accepted a proton from the HCl molecule, so the water has acted as a base.
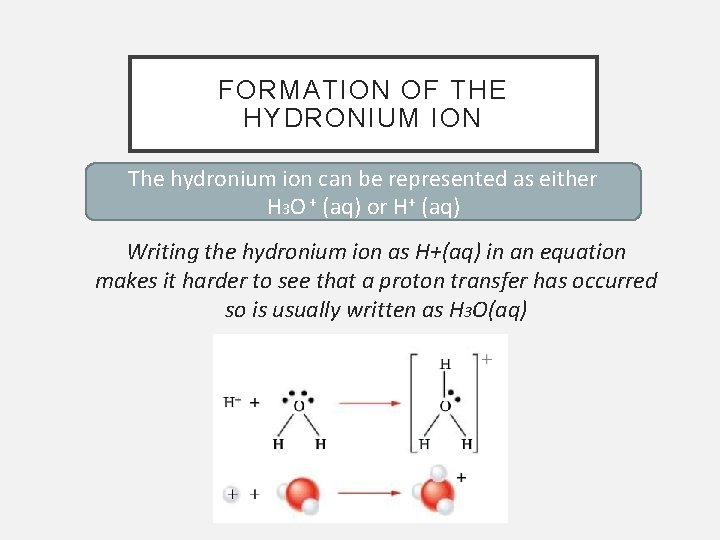
FORMATION OF THE HYDRONIUM ION The hydronium ion can be represented as either H 3 O + (aq) or H+ (aq) Writing the hydronium ion as H+(aq) in an equation makes it harder to see that a proton transfer has occurred so is usually written as H 3 O(aq)
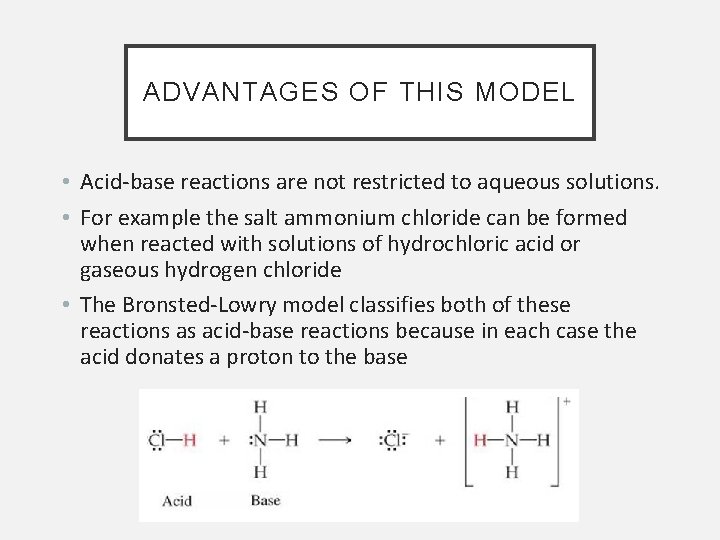
ADVANTAGES OF THIS MODEL • Acid-base reactions are not restricted to aqueous solutions. • For example the salt ammonium chloride can be formed when reacted with solutions of hydrochloric acid or gaseous hydrogen chloride • The Bronsted-Lowry model classifies both of these reactions as acid-base reactions because in each case the acid donates a proton to the base
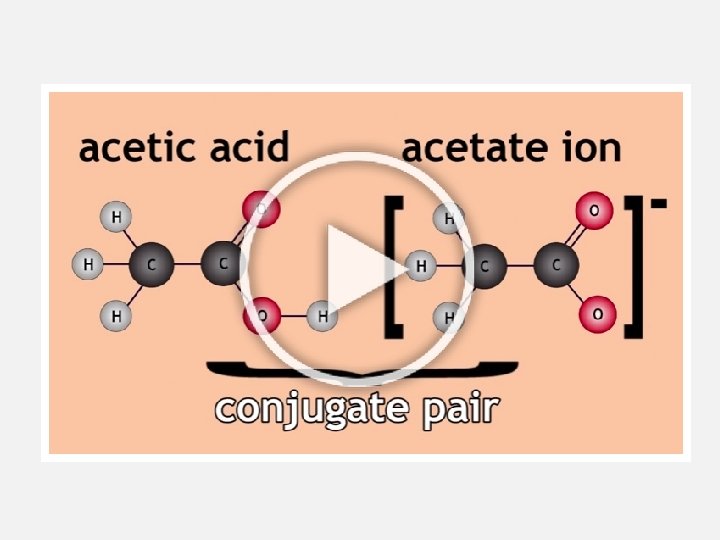
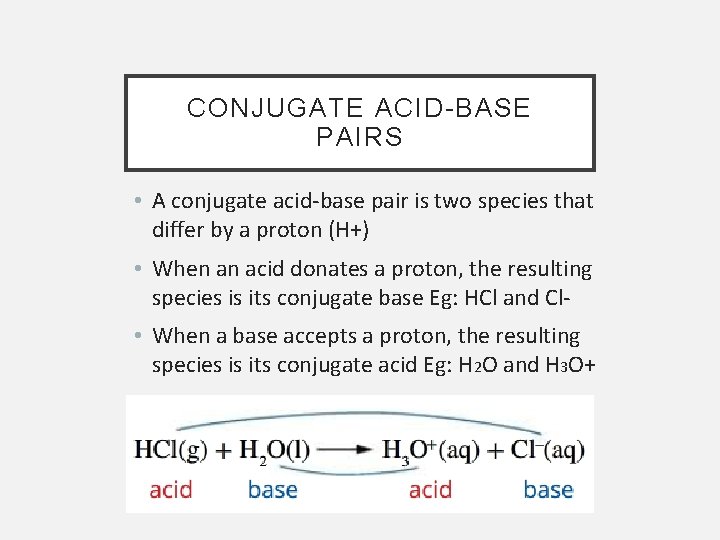
CONJUGATE ACID-BASE PAIRS • A conjugate acid-base pair is two species that differ by a proton (H+) • When an acid donates a proton, the resulting species is its conjugate base Eg: HCl and Cl • When a base accepts a proton, the resulting species is its conjugate acid Eg: H 2 O and H 3 O+
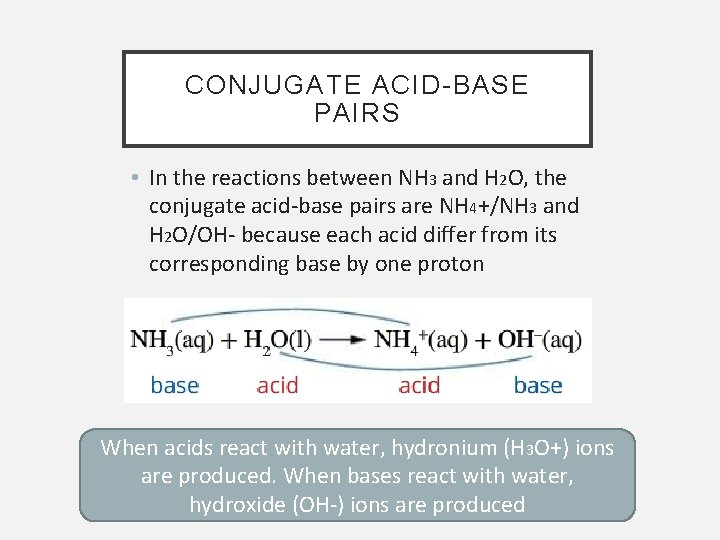
CONJUGATE ACID-BASE PAIRS • In the reactions between NH 3 and H 2 O, the conjugate acid-base pairs are NH 4+/NH 3 and H 2 O/OH- because each acid differ from its corresponding base by one proton When acids react with water, hydronium (H 3 O+) ions are produced. When bases react with water, hydroxide (OH-) ions are produced
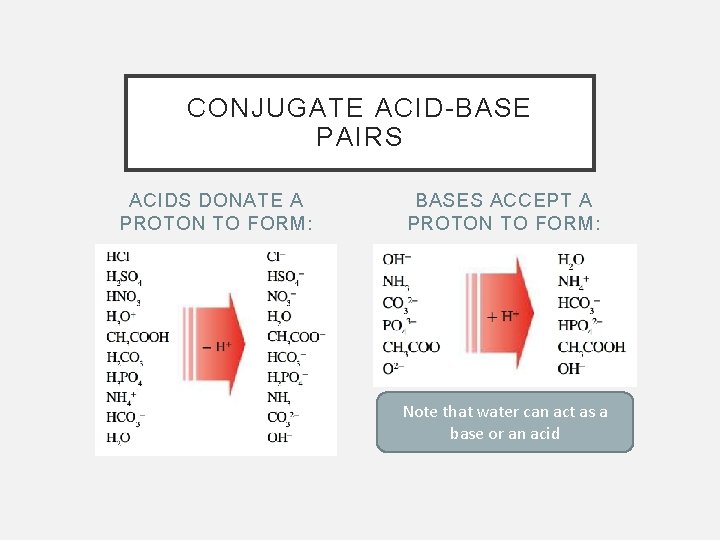
CONJUGATE ACID-BASE PAIRS ACIDS DONATE A PROTON TO FORM: BASES ACCEPT A PROTON TO FORM: Note that water can act as a base or an acid
AMPHIPROTIC SUBSTANCES • Some substances can donate or accept protons depending on what they are reacting with • They can behave as either an acid or a base
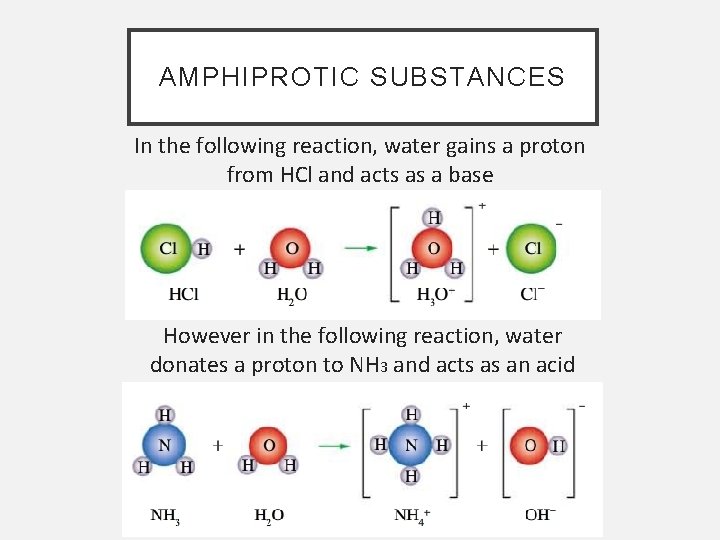
AMPHIPROTIC SUBSTANCES In the following reaction, water gains a proton from HCl and acts as a base However in the following reaction, water donates a proton to NH 3 and acts as an acid
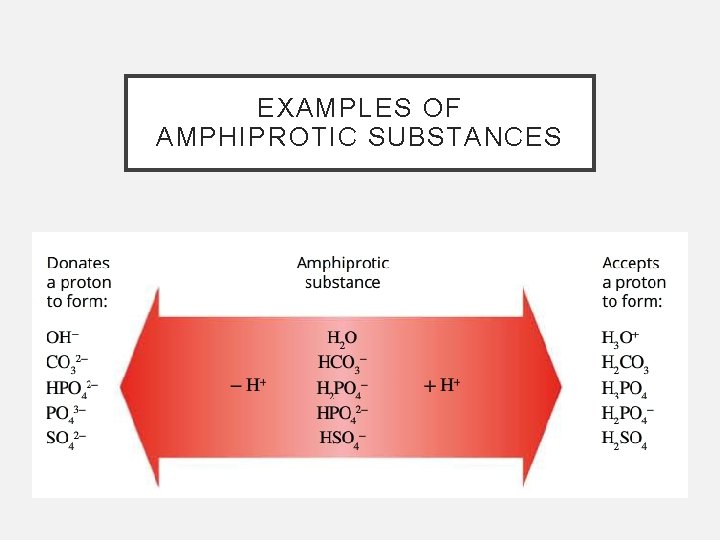
EXAMPLES OF AMPHIPROTIC SUBSTANCES
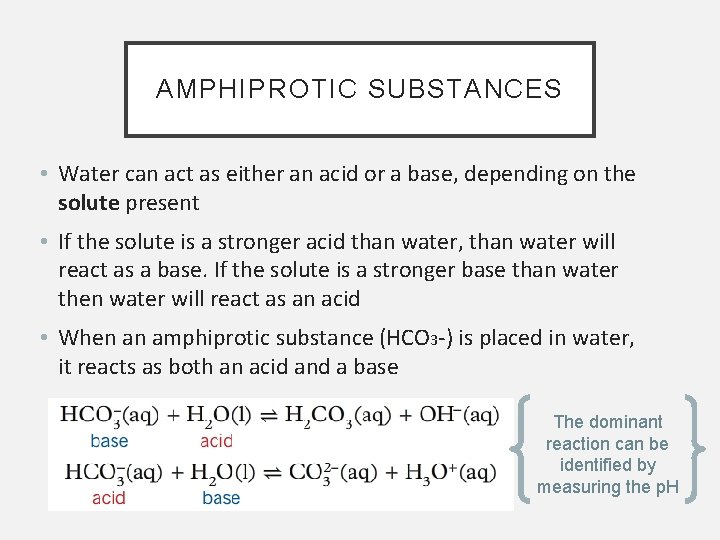
AMPHIPROTIC SUBSTANCES • Water can act as either an acid or a base, depending on the solute present • If the solute is a stronger acid than water, than water will react as a base. If the solute is a stronger base than water then water will react as an acid • When an amphiprotic substance (HCO 3 -) is placed in water, it reacts as both an acid and a base The dominant reaction can be identified by measuring the p. H
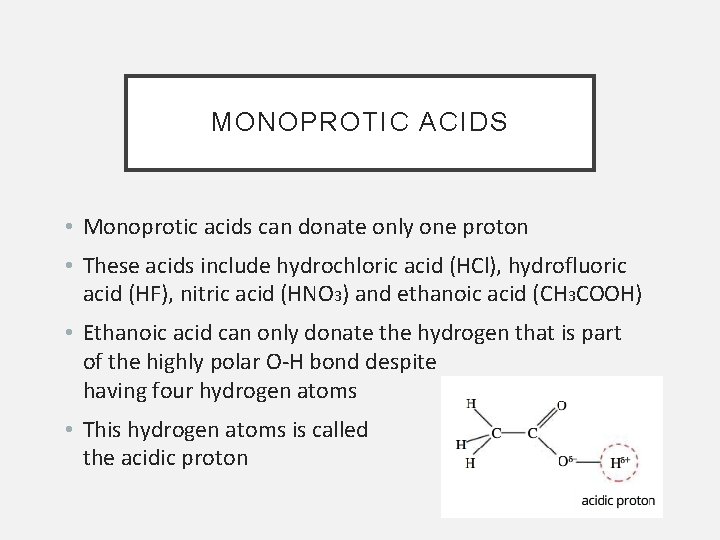
MONOPROTIC ACIDS • Monoprotic acids can donate only one proton • These acids include hydrochloric acid (HCl), hydrofluoric acid (HF), nitric acid (HNO 3) and ethanoic acid (CH 3 COOH) • Ethanoic acid can only donate the hydrogen that is part of the highly polar O-H bond despite having four hydrogen atoms • This hydrogen atoms is called the acidic proton
POLYPROTIC ACIDS • Polyprotic acids can donate more than one proton. The number of hydrogen ions an acid can donate depends on the structure of the acid. • Polyprotic acids do not donate all of their protons at once, they do so in steps when reacting with a base • Diprotic acids can donate two protons (eg: sulphuric acid (H 2 SO 4) and carbonic acid (H 2 CO 3) • Triprotic acids can donate three protons (eg: phosphoric acid (H 3 PO 4) and boric acid (H 3 BO 3)
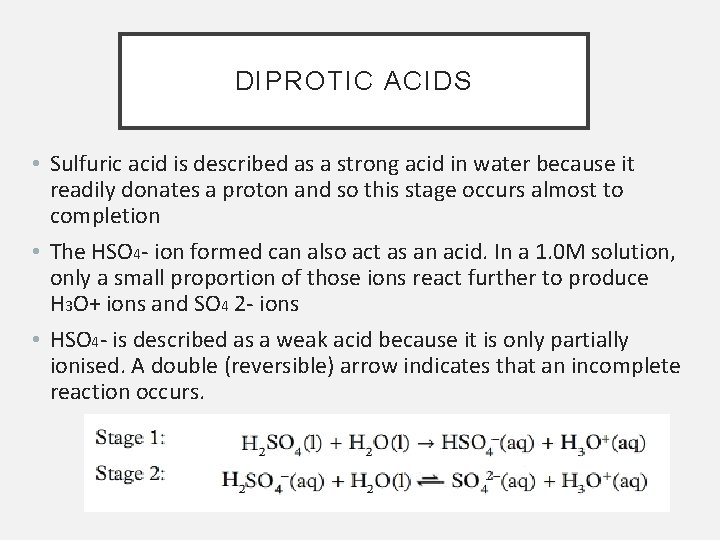
DIPROTIC ACIDS • Sulfuric acid is described as a strong acid in water because it readily donates a proton and so this stage occurs almost to completion • The HSO 4 - ion formed can also act as an acid. In a 1. 0 M solution, only a small proportion of those ions react further to produce H 3 O+ ions and SO 4 2 - ions • HSO 4 - is described as a weak acid because it is only partially ionised. A double (reversible) arrow indicates that an incomplete reaction occurs.
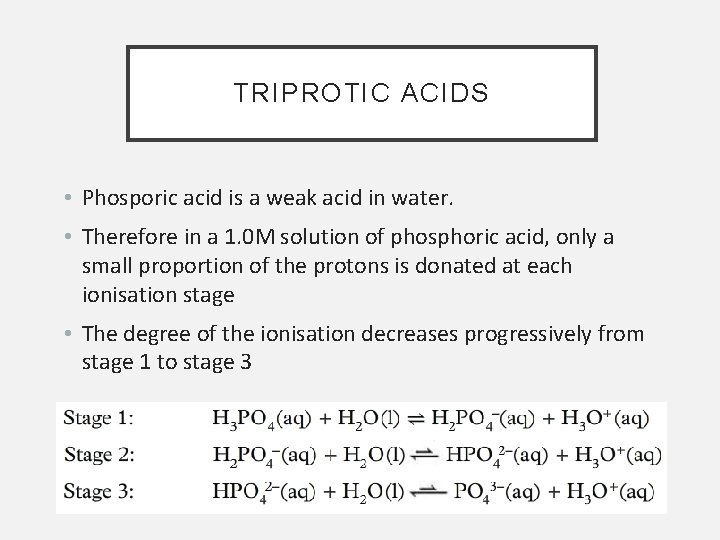
TRIPROTIC ACIDS • Phosporic acid is a weak acid in water. • Therefore in a 1. 0 M solution of phosphoric acid, only a small proportion of the protons is donated at each ionisation stage • The degree of the ionisation decreases progressively from stage 1 to stage 3

STRENGTH OF ACIDS AND BASES Chapter 8 A
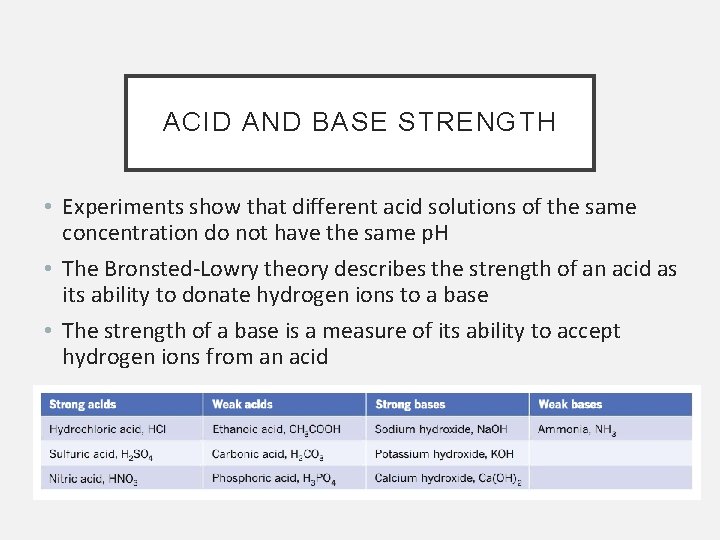
ACID AND BASE STRENGTH • Experiments show that different acid solutions of the same concentration do not have the same p. H • The Bronsted-Lowry theory describes the strength of an acid as its ability to donate hydrogen ions to a base • The strength of a base is a measure of its ability to accept hydrogen ions from an acid
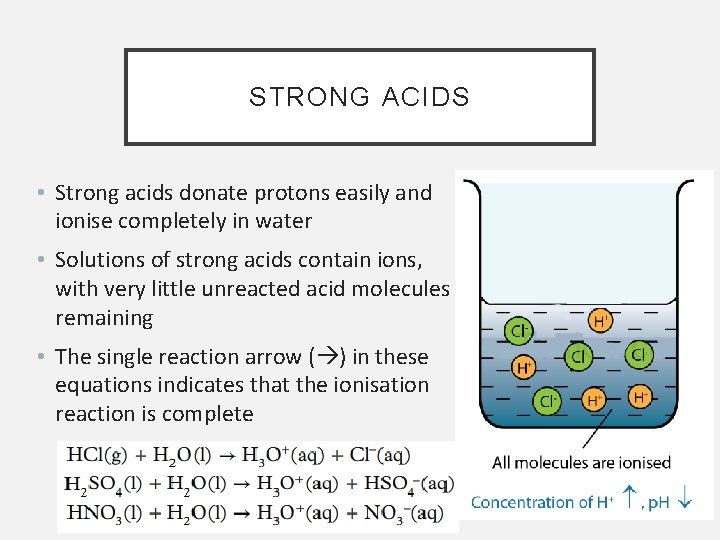
STRONG ACIDS • Strong acids donate protons easily and ionise completely in water • Solutions of strong acids contain ions, with very little unreacted acid molecules remaining • The single reaction arrow ( ) in these equations indicates that the ionisation reaction is complete
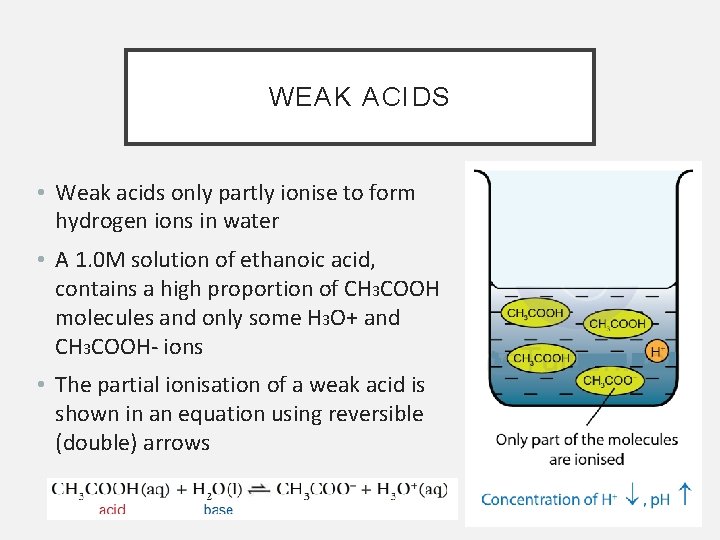
WEAK ACIDS • Weak acids only partly ionise to form hydrogen ions in water • A 1. 0 M solution of ethanoic acid, contains a high proportion of CH 3 COOH molecules and only some H 3 O+ and CH 3 COOH- ions • The partial ionisation of a weak acid is shown in an equation using reversible (double) arrows
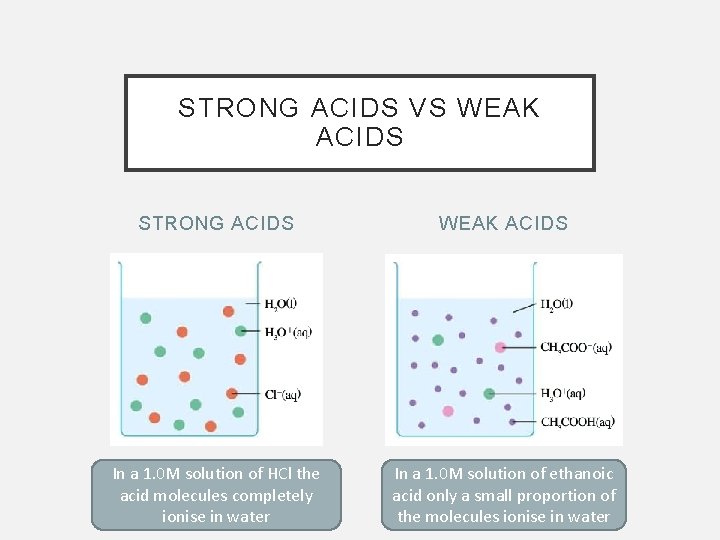
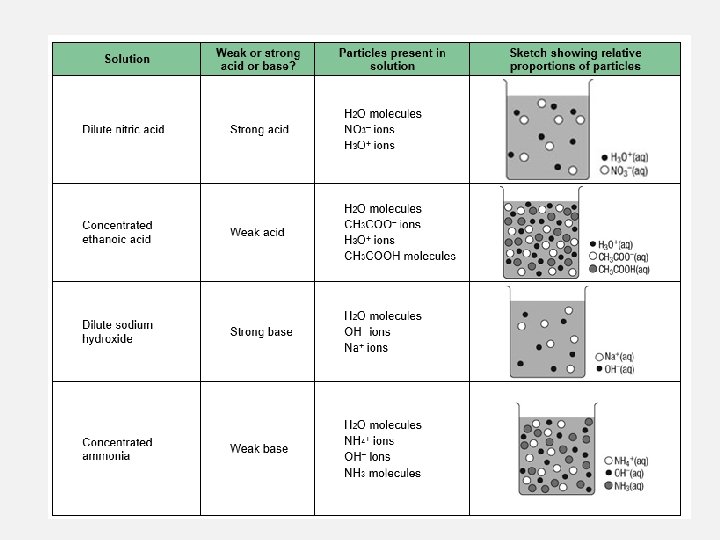
STRONG ACIDS VS WEAK ACIDS STRONG ACIDS WEAK ACIDS In a 1. 0 M solution of HCl the acid molecules completely ionise in water In a 1. 0 M solution of ethanoic acid only a small proportion of the molecules ionise in water
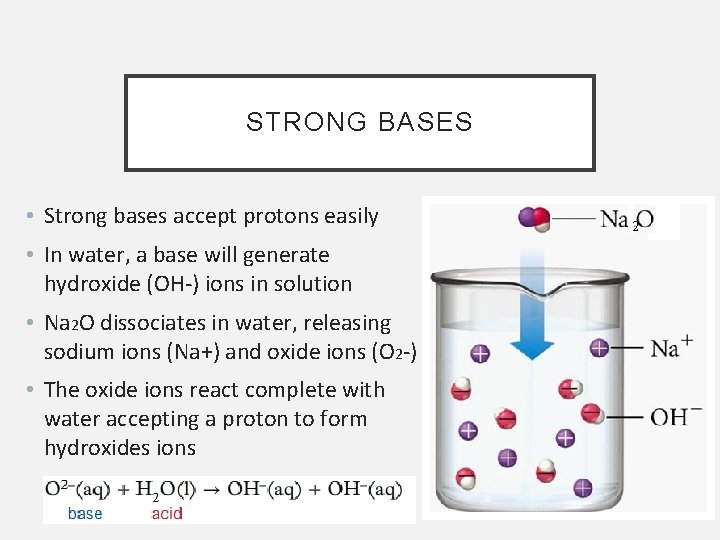
STRONG BASES • Strong bases accept protons easily • In water, a base will generate hydroxide (OH-) ions in solution • Na 2 O dissociates in water, releasing sodium ions (Na+) and oxide ions (O 2 -) • The oxide ions react complete with water accepting a proton to form hydroxides ions 2
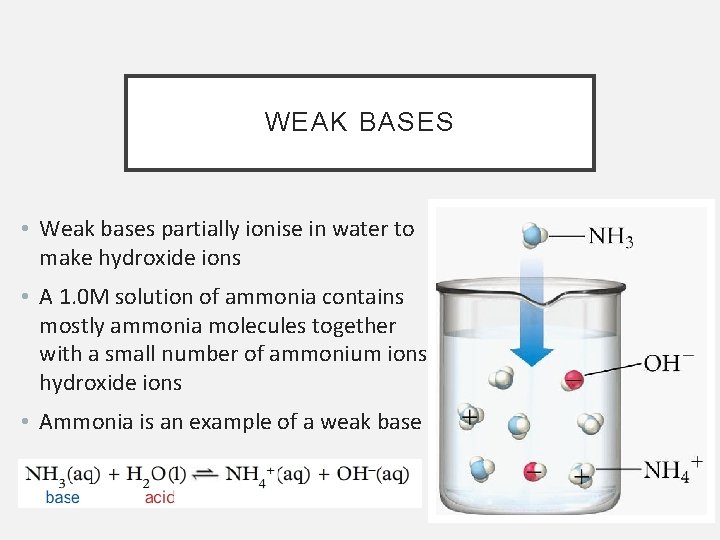
WEAK BASES • Weak bases partially ionise in water to make hydroxide ions • A 1. 0 M solution of ammonia contains mostly ammonia molecules together with a small number of ammonium ions and hydroxide ions • Ammonia is an example of a weak base
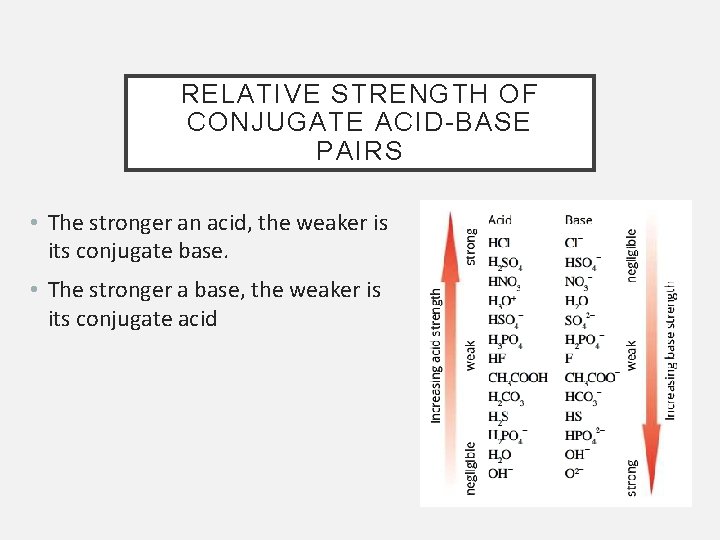
RELATIVE STRENGTH OF CONJUGATE ACID-BASE PAIRS • The stronger an acid, the weaker is its conjugate base. • The stronger a base, the weaker is its conjugate acid
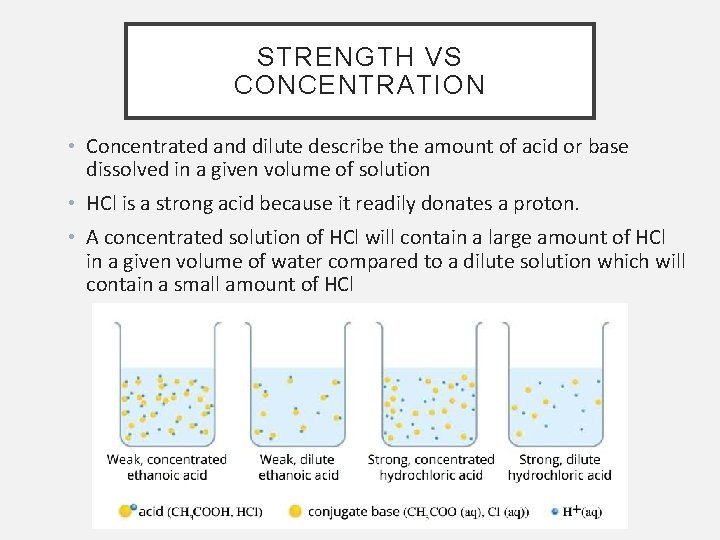
STRENGTH VS CONCENTRATION • Concentrated and dilute describe the amount of acid or base dissolved in a given volume of solution • HCl is a strong acid because it readily donates a proton. • A concentrated solution of HCl will contain a large amount of HCl in a given volume of water compared to a dilute solution which will contain a small amount of HCl
PH SCALE AND INDICATORS Chapter 8 B

PH OF ACIDIC AND BASIC SOLUTIONS • Acidic, basic and neutral solutions can be defined in terms of the p. H at 25°C Neutral solution have a p. H equal to 7 Acidic solutions have a p. H less than 7 Basic solutions have a p. H greater than 7 • A solution with a p. H of 2 has 10 times the concentration of H 3 O+ ions as one of p. H of 3
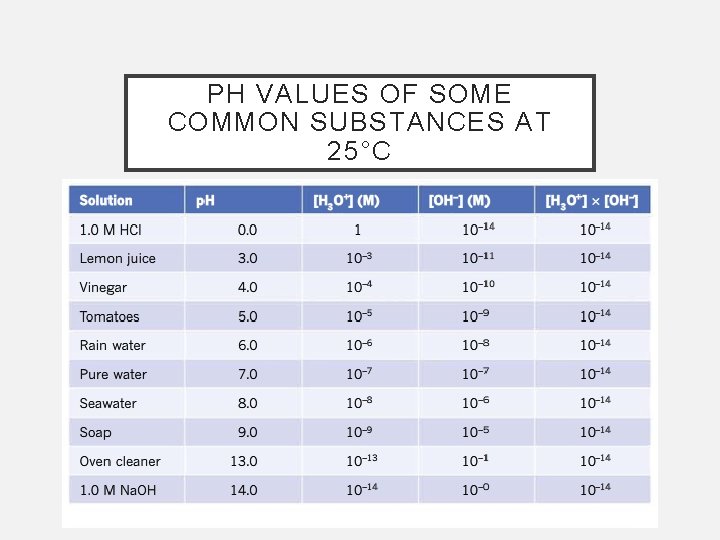
PH VALUES OF SOME COMMON SUBSTANCES AT 25°C
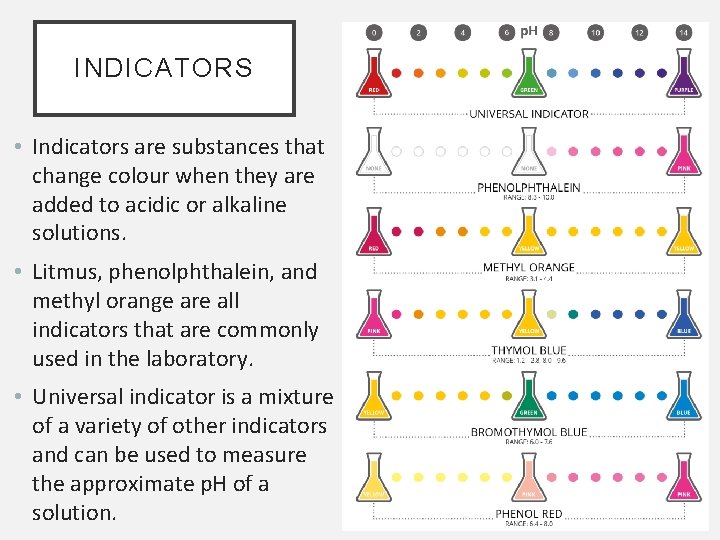
INDICATORS • Indicators are substances that change colour when they are added to acidic or alkaline solutions. • Litmus, phenolphthalein, and methyl orange are all indicators that are commonly used in the laboratory. • Universal indicator is a mixture of a variety of other indicators and can be used to measure the approximate p. H of a solution.
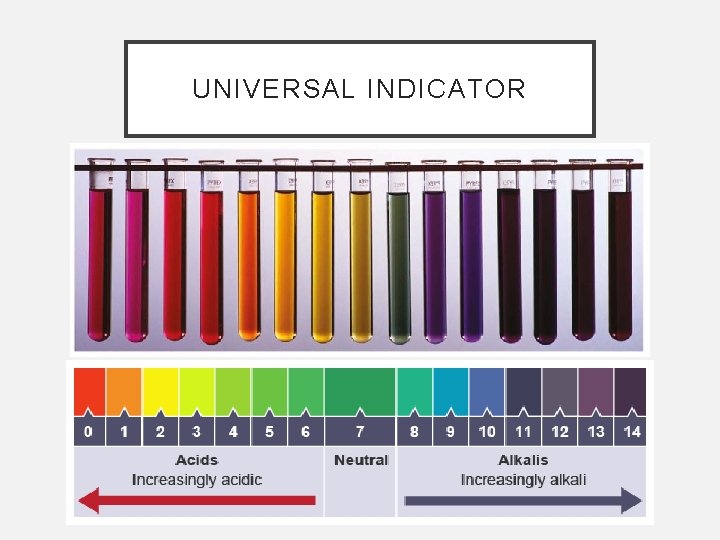
UNIVERSAL INDICATOR


DILUTION OF ACIDS AND BASES

CONCENTRATION OF ACIDS AND BASES • The concentration of acids and bases is usually expressed in units of mol/L or M also known as molarity. • The most convenient way of preparing a solution of a dilute acid is by mixing concentrated acid with water. This is known as dilution. c 1 V 1 = c 2 V 2
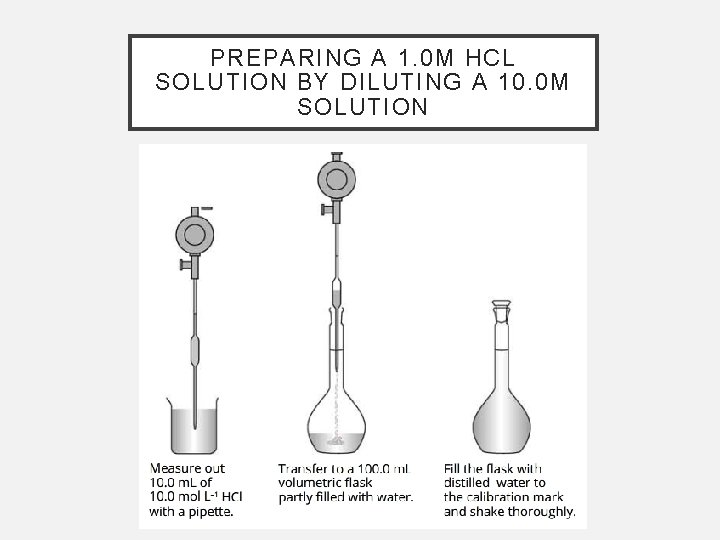
PREPARING A 1. 0 M HCL SOLUTION BY DILUTING A 10. 0 M SOLUTION
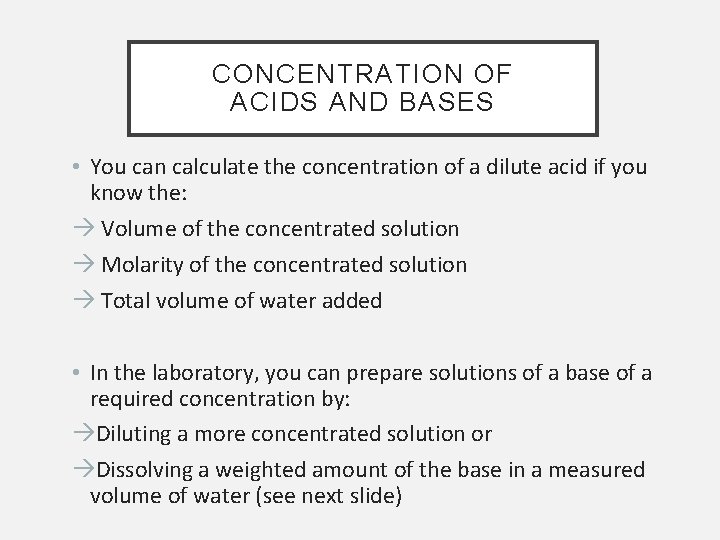
CONCENTRATION OF ACIDS AND BASES • You can calculate the concentration of a dilute acid if you know the: Volume of the concentrated solution Molarity of the concentrated solution Total volume of water added • In the laboratory, you can prepare solutions of a base of a required concentration by: Diluting a more concentrated solution or Dissolving a weighted amount of the base in a measured volume of water (see next slide)
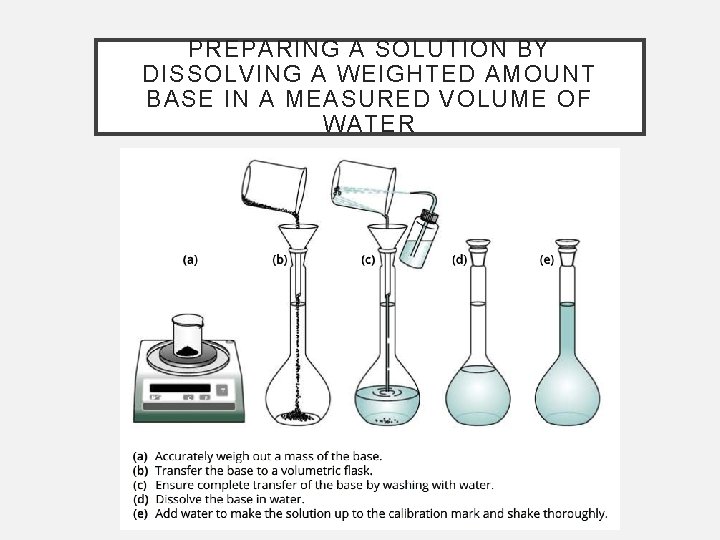
PREPARING A SOLUTION BY DISSOLVING A WEIGHTED AMOUNT BASE IN A MEASURED VOLUME OF WATER
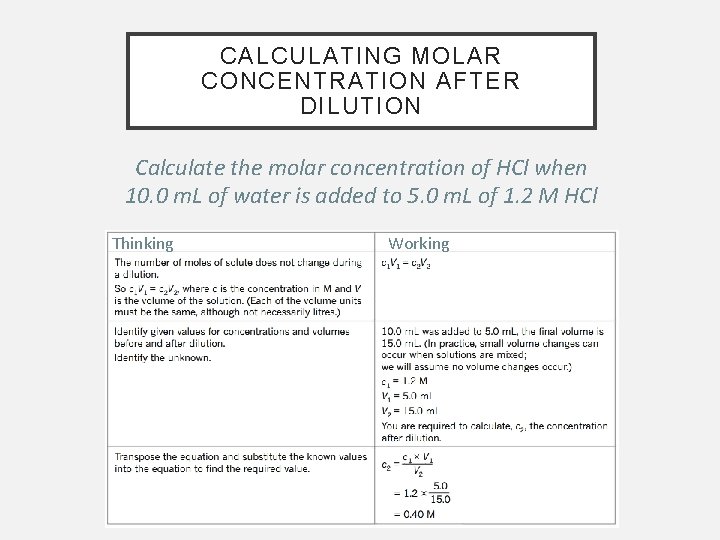
CALCULATING MOLAR CONCENTRATION AFTER DILUTION Calculate the molar concentration of HCl when 10. 0 m. L of water is added to 5. 0 m. L of 1. 2 M HCl Thinking Working
YOUR TURN Calculate the molar concentration of HNO 3 when 80. 0 m. L of water is added to 20. 0 m. L of 5. 00 M HNO 3

CALCULATING THE VOLUME OF WATER TO BE ADDED IN A DILUTION How much water must be added to 30. 0 m. L of 2. 50 M HCl to dilute the solution to 1. 00 M? Thinking Working

YOUR TURN How much water must be added to 15. 0 m. L of 10. 0 M Na. OH to dilute the solution to 2. 00 M?

EFFECT OF DILUTION ON PH OF STRONG ACIDS AND BASES • Consider a 0. 1 M solution of HCl is a strong acid so the concentration of H 3 O+ ions is 0. 1 M Since p. H = -log 10[H 3 O+], the p. H of this solution is 1. 0 • If this 1. 0 m. L solution is diluted by a factor is 10 to 10. 0 m. L by the addition of 9. 0 m. L of water, the concentration of H 3 O+ ions decreases to 0. 01 M and the p. H increases to 2. 0 • A further dilution by a factor of 10 to 100 m. L will increase p. H to 3. 0. However when acids are repeatedly diluted, the p. H cannot increase above 7

REACTIONS OF ACIDS AND BASES Chapter 8 C
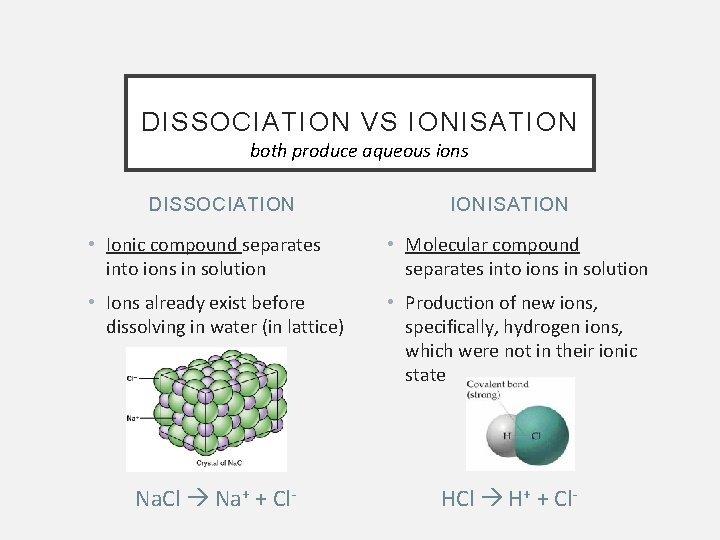
DISSOCIATION VS IONISATION both produce aqueous ions DISSOCIATION IONISATION • Ionic compound separates into ions in solution • Molecular compound separates into ions in solution • Ions already exist before dissolving in water (in lattice) • Production of new ions, specifically, hydrogen ions, which were not in their ionic state Na. Cl Na+ + Cl- HCl H+ + Cl-
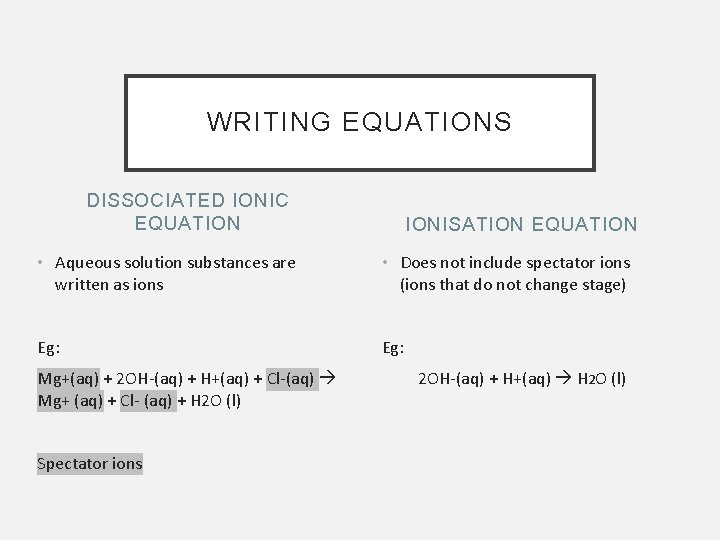
WRITING EQUATIONS DISSOCIATED IONIC EQUATION IONISATION EQUATION • Aqueous solution substances are written as ions • Does not include spectator ions (ions that do not change stage) Eg: Mg+(aq) + 2 OH-(aq) + H+(aq) + Cl-(aq) Mg+ (aq) + Cl- (aq) + H 2 O (l) Spectator ions 2 OH-(aq) + H+(aq) H 2 O (l)
GENERAL REACTION TYPES INVOLVING ACIDS AND BASES • Acids and bases react in many ways. It is possible to group some reactions together according to the similarity of reactants and products formed. • The reaction types you will be studying are the reaction of acids with: metal hydroxides metal carbonates and hydrogen carbonates reactive metals
ACIDS AND METAL HYDROXIDES • Soluble metal oxides, such as Na. OH, dissociate in water to form metal cations and hydroxide ions • The products of a reaction of an acid with a metal hydroxide are an ionic compound called a salt and water acid + metal hydroxide salt + water • For example: solutions of sulphuric acid and sodium hydroxide react to form sodium sulfate and water
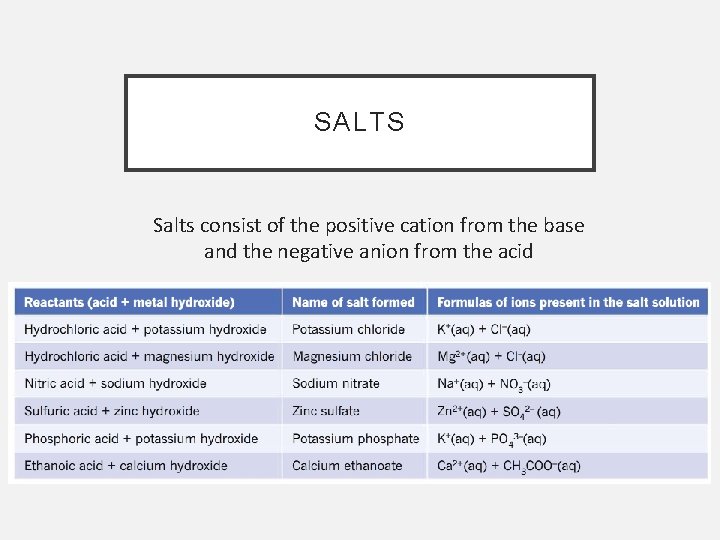
SALTS Salts consist of the positive cation from the base and the negative anion from the acid
IONIC EQUATIONS When writing ionic equations remember that: Ionic equations are balanced with respect to both the number of atoms of each element and charge Spectator ions are ions that are dissolved in the solution and are not involved in the reaction If a reactant or product is a solid, liquid or gas, it cannot be written as ions and it must be present in the ionic equation

IONIC EQUATIONS You also need to remember when writing ionic equations for neutralisation reactions: Strong acids ionise in solution and are written as ions eg: HCl in solution is written as H+ (aq) and Cl-(aq) Metal hydroxides and salts are ionic and if soluble, dissociate in solution and are written as ions eg: KOH dissolving in water is written as KOH(s) K+(aq) + OH-(aq) Water is a covalent molecular substance that does not ionise to and significant extent and is written as H 2 O(l)
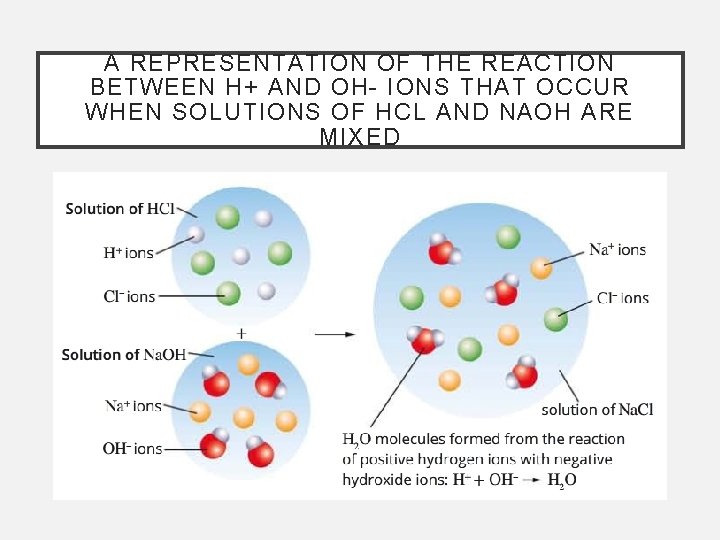
A REPRESENTATION OF THE REACTION BETWEEN H+ AND OH- IONS THAT OCCUR WHEN SOLUTIONS OF HCL AND NAOH ARE MIXED
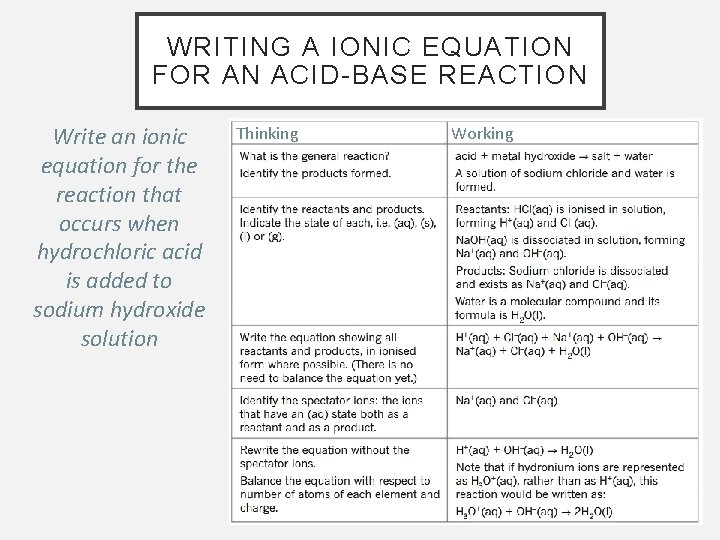
WRITING A IONIC EQUATION FOR AN ACID-BASE REACTION Write an ionic equation for the reaction that occurs when hydrochloric acid is added to sodium hydroxide solution Thinking Working

YOUR TURN Write an ionic equation for the reaction that occurs when sulfuric acid is added to potassium hydroxide solution
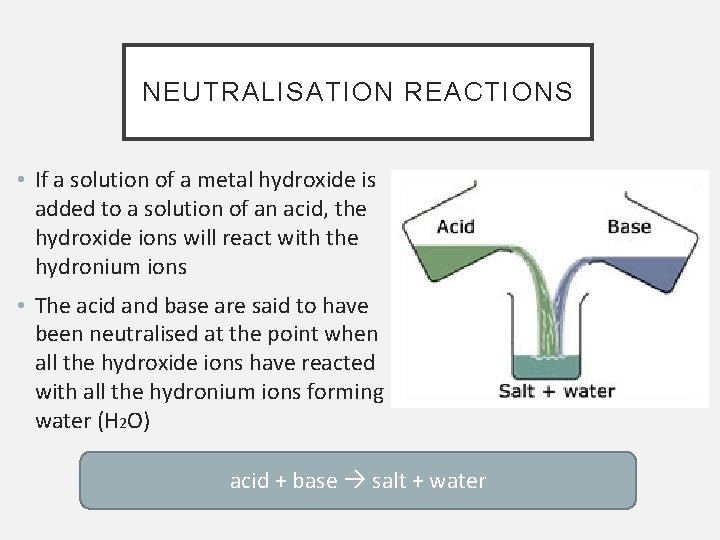
NEUTRALISATION REACTIONS • If a solution of a metal hydroxide is added to a solution of an acid, the hydroxide ions will react with the hydronium ions • The acid and base are said to have been neutralised at the point when all the hydroxide ions have reacted with all the hydronium ions forming water (H 2 O) acid + base salt + water
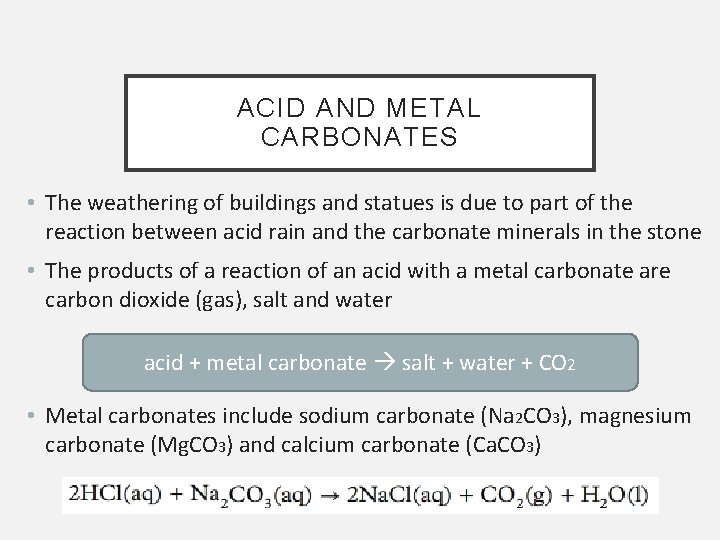
ACID AND METAL CARBONATES • The weathering of buildings and statues is due to part of the reaction between acid rain and the carbonate minerals in the stone • The products of a reaction of an acid with a metal carbonate are carbon dioxide (gas), salt and water acid + metal carbonate salt + water + CO 2 • Metal carbonates include sodium carbonate (Na 2 CO 3), magnesium carbonate (Mg. CO 3) and calcium carbonate (Ca. CO 3)
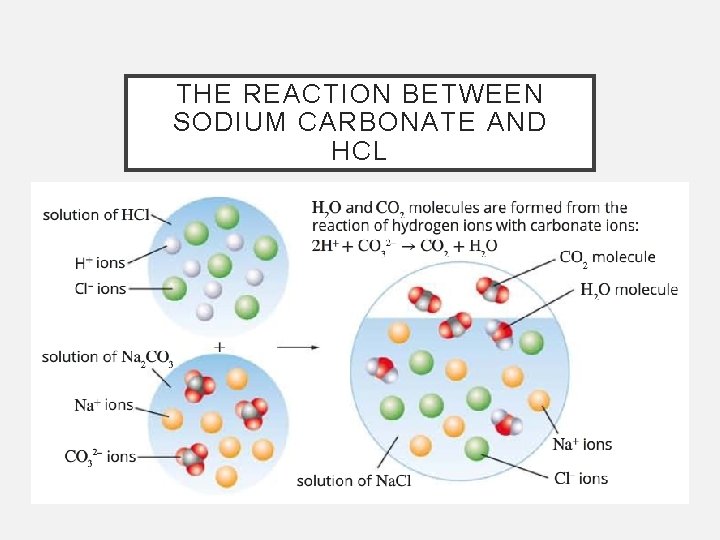
THE REACTION BETWEEN SODIUM CARBONATE AND HCL
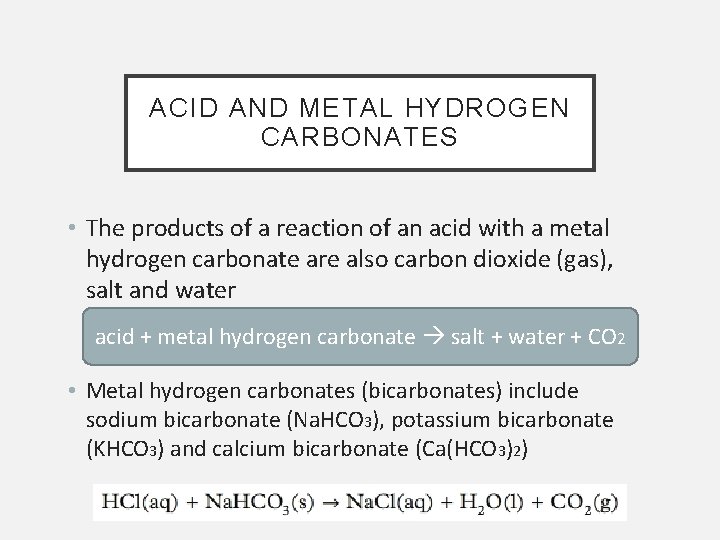
ACID AND METAL HYDROGEN CARBONATES • The products of a reaction of an acid with a metal hydrogen carbonate are also carbon dioxide (gas), salt and water acid + metal hydrogen carbonate salt + water + CO 2 • Metal hydrogen carbonates (bicarbonates) include sodium bicarbonate (Na. HCO 3), potassium bicarbonate (KHCO 3) and calcium bicarbonate (Ca(HCO 3)2)
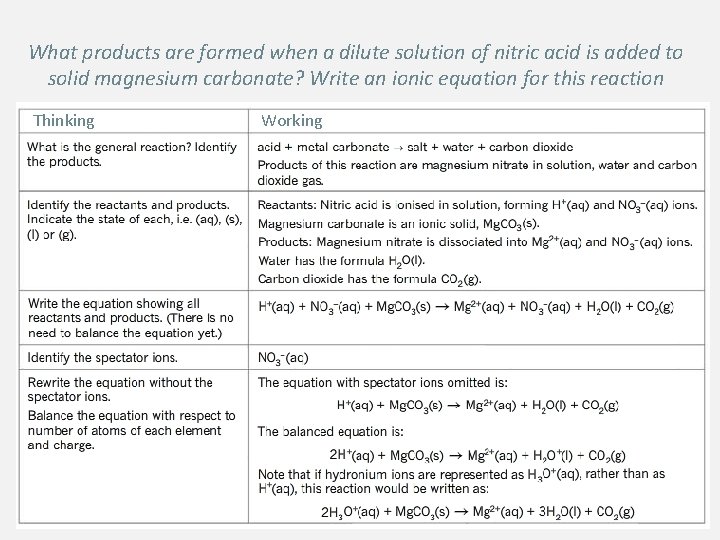
What products are formed when a dilute solution of nitric acid is added to solid magnesium carbonate? Write an ionic equation for this reaction Thinking Working

YOUR TURN What products are formed when a solution of hydrochloric acid is added to a solution of sodium hydrogen carbonate? Write an ionic equation for this reaction
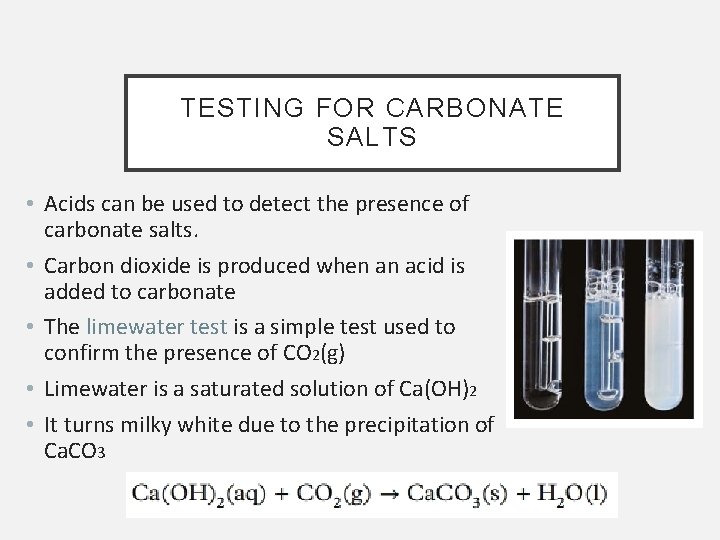
TESTING FOR CARBONATE SALTS • Acids can be used to detect the presence of carbonate salts. • Carbon dioxide is produced when an acid is added to carbonate • The limewater test is a simple test used to confirm the presence of CO 2(g) • Limewater is a saturated solution of Ca(OH)2 • It turns milky white due to the precipitation of Ca. CO 3

ACIDS AND REACTIVE METALS • When dilute acids are added to main group metals and some transition metals, bubbles of hydrogen gas are released and a salt is formed acid + reactive metal salt + hydrogen gas • Reactive metals include calcium, magnesium, iron, zinc but not copper, silver or gold.
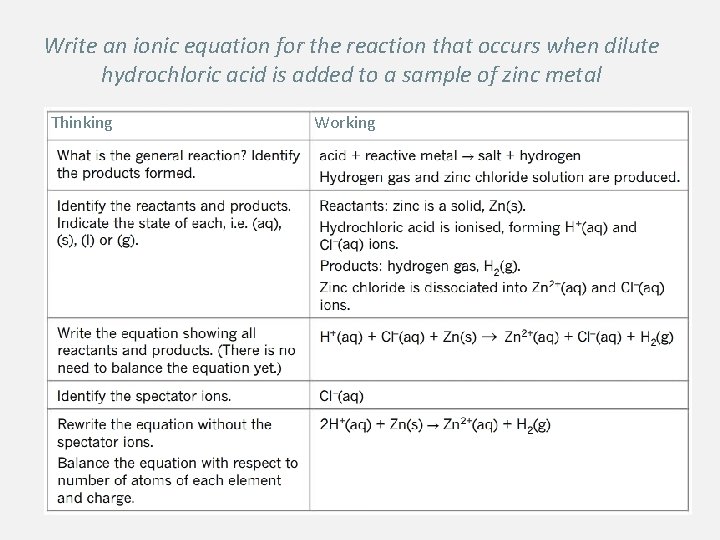
Write an ionic equation for the reaction that occurs when dilute hydrochloric acid is added to a sample of zinc metal Thinking Working

YOUR TURN Write an ionic equation for the reaction that occurs when aluminium is added to a solution of hydrochloric acid
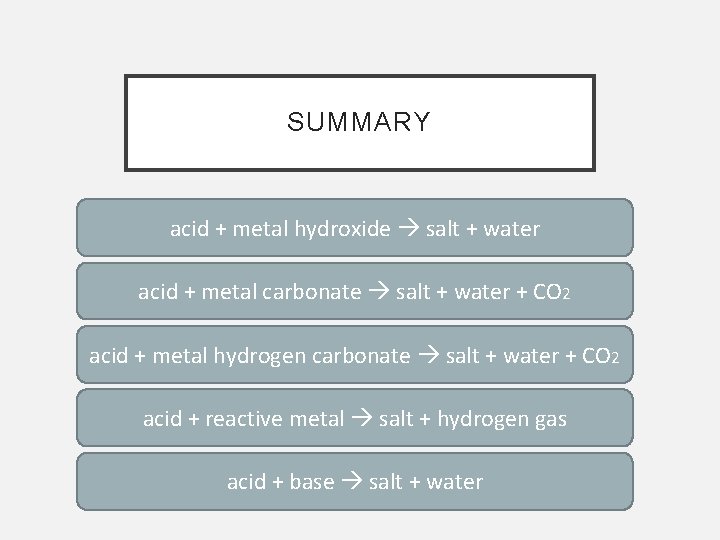
SUMMARY acid + metal hydroxide salt + water acid + metal carbonate salt + water + CO 2 acid + metal hydrogen carbonate salt + water + CO 2 acid + reactive metal salt + hydrogen gas acid + base salt + water
- Slides: 75