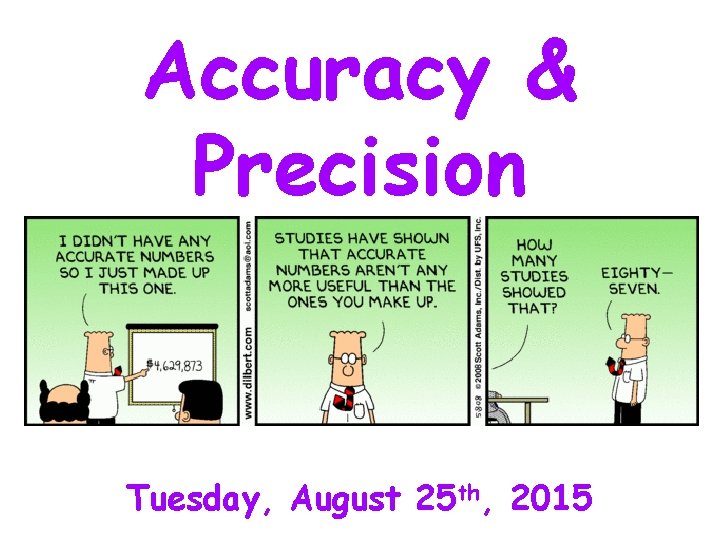
Accuracy Precision Tuesday August 25 th 2015 Accuracy

- Slides: 9

Accuracy & Precision Tuesday, August 25 th, 2015

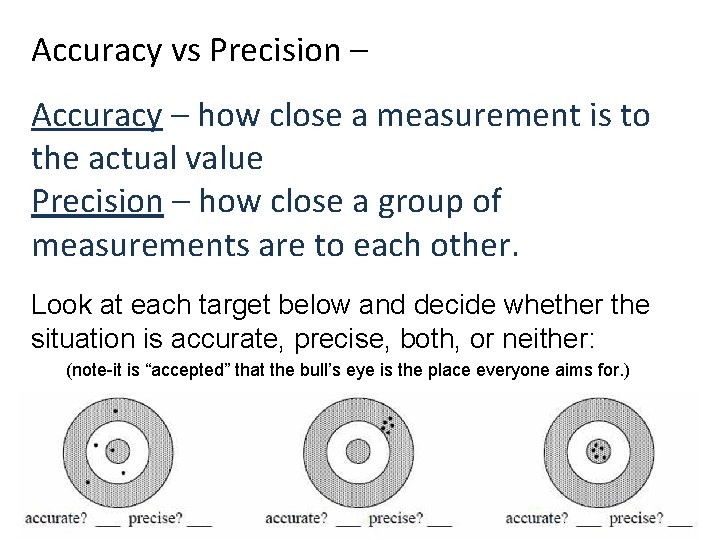
Accuracy vs Precision – Accuracy – how close a measurement is to the actual value Precision – how close a group of measurements are to each other. Look at each target below and decide whether the situation is accurate, precise, both, or neither: (note-it is “accepted” that the bull’s eye is the place everyone aims for. )

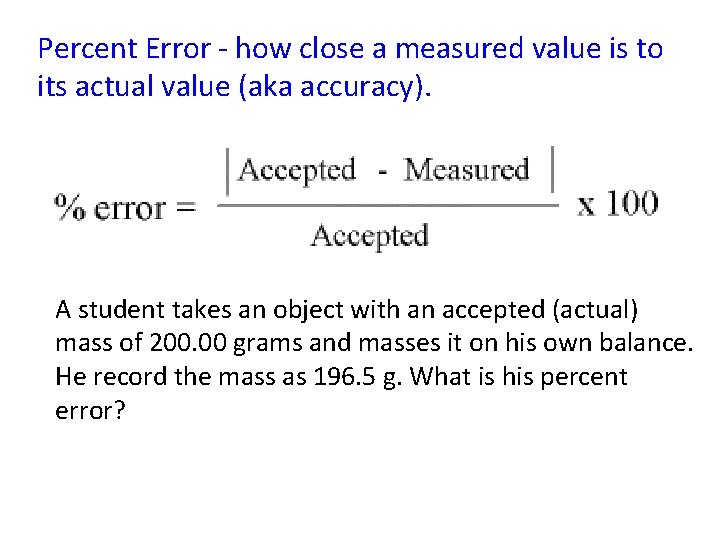
Percent Error - how close a measured value is to its actual value (aka accuracy). A student takes an object with an accepted (actual) mass of 200. 00 grams and masses it on his own balance. He record the mass as 196. 5 g. What is his percent error?

How accurate do you need to be. . +/- 10%

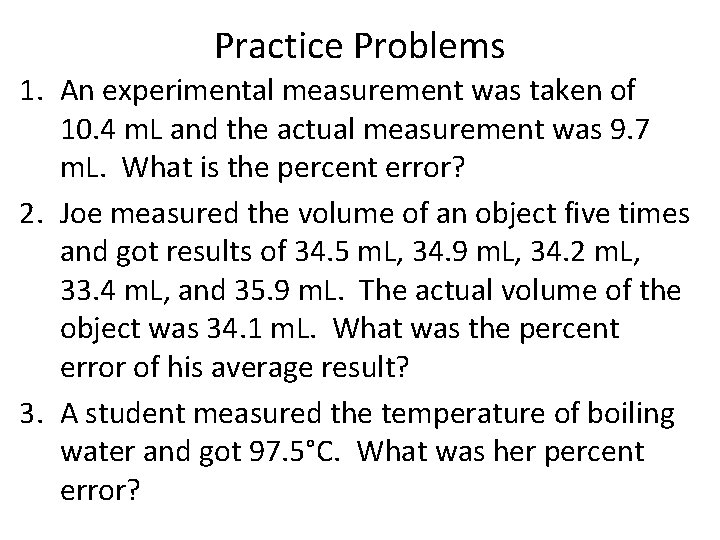
Practice Problems 1. An experimental measurement was taken of 10. 4 m. L and the actual measurement was 9. 7 m. L. What is the percent error? 2. Joe measured the volume of an object five times and got results of 34. 5 m. L, 34. 9 m. L, 34. 2 m. L, 33. 4 m. L, and 35. 9 m. L. The actual volume of the object was 34. 1 m. L. What was the percent error of his average result? 3. A student measured the temperature of boiling water and got 97. 5°C. What was her percent error?

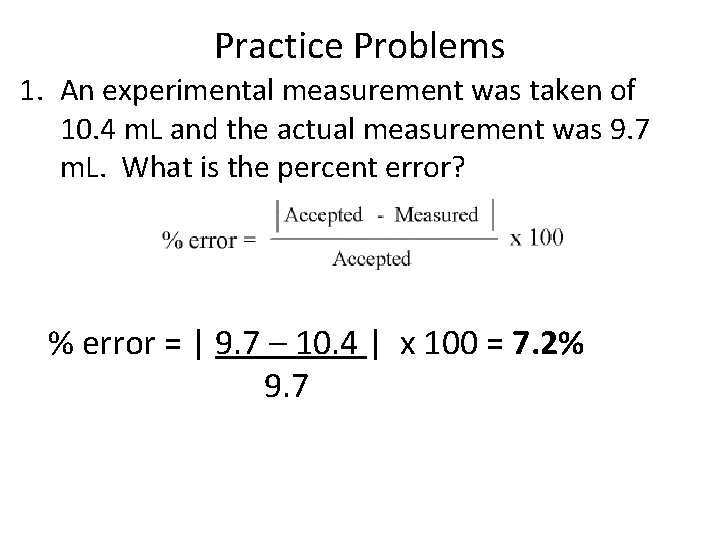
Practice Problems 1. An experimental measurement was taken of 10. 4 m. L and the actual measurement was 9. 7 m. L. What is the percent error? % error = | 9. 7 – 10. 4 | x 100 = 7. 2% 9. 7

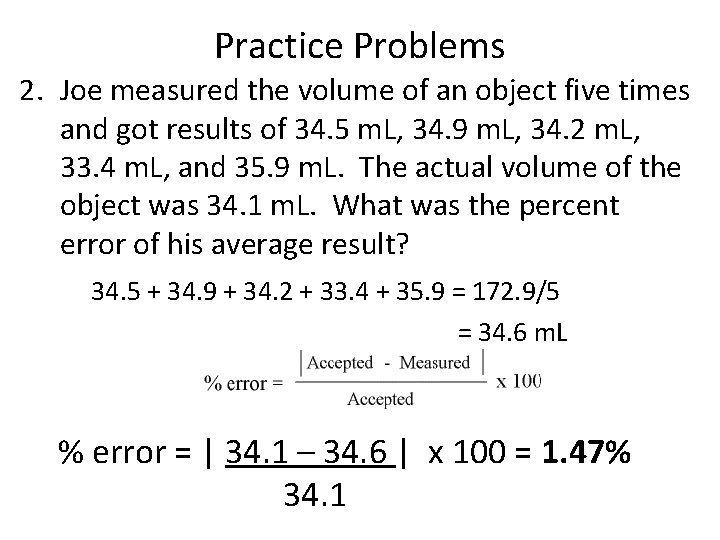
Practice Problems 2. Joe measured the volume of an object five times and got results of 34. 5 m. L, 34. 9 m. L, 34. 2 m. L, 33. 4 m. L, and 35. 9 m. L. The actual volume of the object was 34. 1 m. L. What was the percent error of his average result? 34. 5 + 34. 9 + 34. 2 + 33. 4 + 35. 9 = 172. 9/5 = 34. 6 m. L % error = | 34. 1 – 34. 6 | x 100 = 1. 47% 34. 1

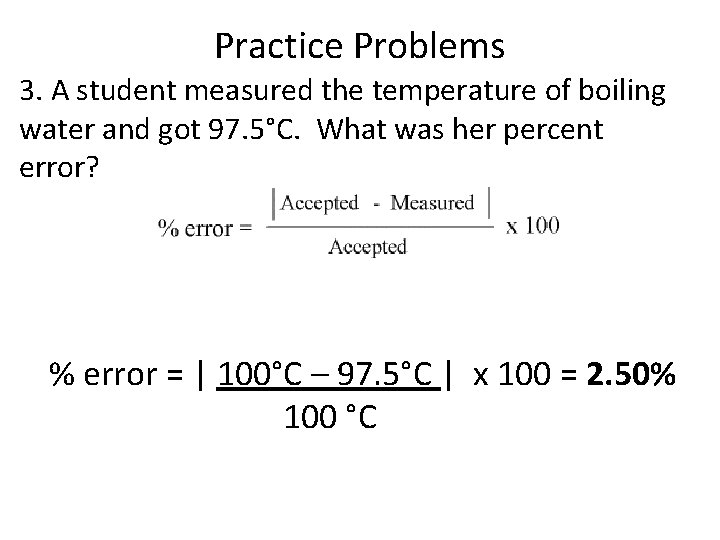
Practice Problems 3. A student measured the temperature of boiling water and got 97. 5°C. What was her percent error? % error = | 100°C – 97. 5°C | x 100 = 2. 50% 100 °C
