ACCREDITATION PROCESS Mpho Phaloane Introduction Who is SANAS







- Slides: 12

ACCREDITATION PROCESS Mpho Phaloane

Introduction Who is SANAS? • The South African National Accreditation System • SANAS is one of the internationally recognized, national accreditation bodies in the world. It was inaugurated in 1996 section 21 company). (as a • The creation of a single national accreditation body, SANAS, allowed S. A to remain competitive nationally and internationally due to the ability to independently confirm competence of its Technical Infrastructure • Accreditation is increasingly being used by S. A Regulators, as part of managing local regulatory risk, to ensure both the competence and consistency of outcomes of service providers used in the local regulatory domain

Status of SANAS South African National Accreditation System SANAS is recognized as the only national body responsible for carrying out accreditation in respect of conformity assessment, which includes: • Calibration, testing and verification laboratories: • Certification Bodies; • Inspection Bodies; • B-BBEE Rating agencies; and • Monitoring of GLP compliance with principles adopted by the OECD The Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 19 of 2006

1974 – 1996 Limited Company : Non- Profit : Section 21 1974 NCS Calibration Labs 1992 NLA Calibration + Test labs Labs 1996 SANAS Labs, Cert & Insp 2000 OECD - GLP 2007 Public Entity 2007 SANAS Labs, Cert & Insp 2000 OECD – GLP & National Projects



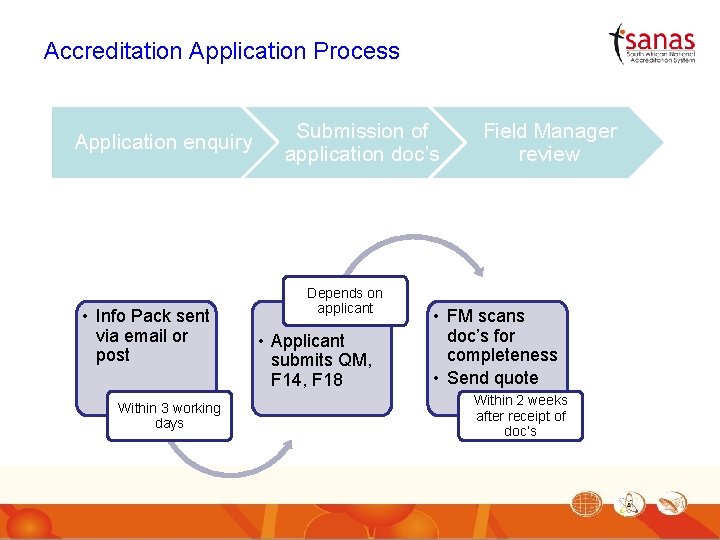
Accreditation Application Process Application enquiry • Info Pack sent via email or post Within 3 working days Submission of application doc’s Depends on applicant • Applicant submits QM, F 14, F 18 Field Manager review • FM scans doc’s for completeness • Send quote Within 2 weeks after receipt of doc’s

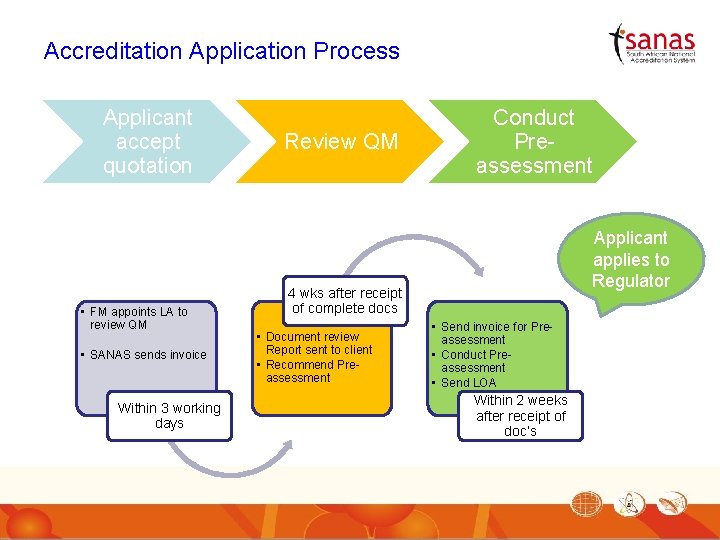
Accreditation Application Process Applicant accept quotation • FM appoints LA to review QM • SANAS sends invoice Within 3 working days Review QM Conduct Preassessment Applicant applies to Regulator 4 wks after receipt of complete docs • Document review Report sent to client • Recommend Preassessment • Send invoice for Preassessment • Conduct Preassessment • Send LOA Within 2 weeks after receipt of doc’s

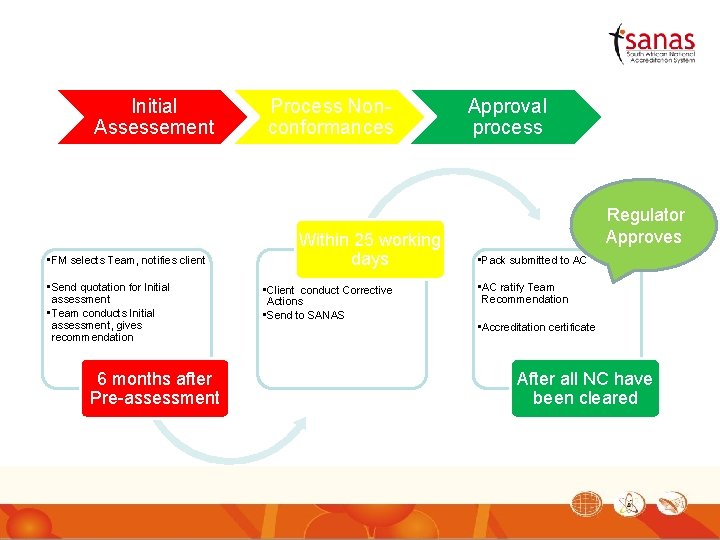
Initial Assessement • FM selects Team, notifies client • Send quotation for Initial assessment • Team conducts Initial assessment, gives recommendation 6 months after Pre-assessment Process Nonconformances Within 25 working days • Client conduct Corrective Actions • Send to SANAS Approval process Regulator Approves • Pack submitted to AC • AC ratify Team Recommendation • Accreditation certificate After all NC have been cleared

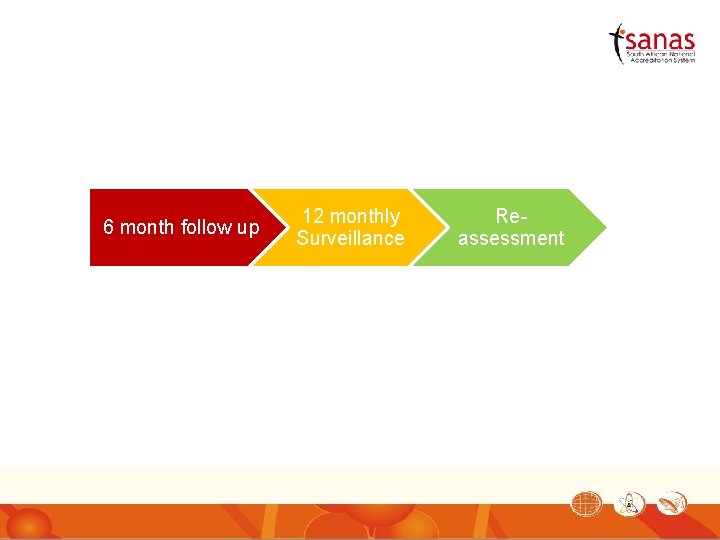
6 month follow up 12 monthly Surveillance Reassessment

Latest status • • Currently only 5 accredited M&V bodies 1 is in advanced stages of accreditation No other applications in process 8 inquiries received since Jan 2015

Thank You Mpho Phaloane Senior Manager: Accreditation Tel. (012) 394 3780 Email: mphop@sanas. co. za Website: www. sanas. co. za
 What is sanas
What is sanas Sanas accreditation process
Sanas accreditation process Sanas r documents
Sanas r documents Nablwp.qci
Nablwp.qci Ward 35 james cook hospital
Ward 35 james cook hospital Uc berkeley extension courses
Uc berkeley extension courses Accsc accreditation good or bad
Accsc accreditation good or bad Fibaa accreditation
Fibaa accreditation International laboratory accreditation cooperation
International laboratory accreditation cooperation Ea european accreditation
Ea european accreditation Acgme accreditation withheld
Acgme accreditation withheld Niaho accreditation
Niaho accreditation Next accreditation system
Next accreditation system






















