Access to the Akuammiline Family of Alkaloids Total






- Slides: 35

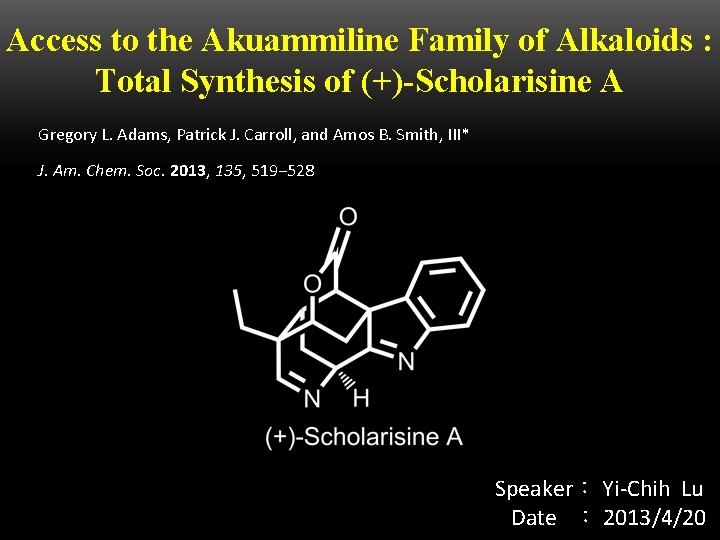
Access to the Akuammiline Family of Alkaloids : Total Synthesis of (+)-Scholarisine A Gregory L. Adams, Patrick J. Carroll, and Amos B. Smith, III* J. Am. Chem. Soc. 2013, 135, 519− 528 Speaker: Yi-Chih Lu Date : 2013/4/20

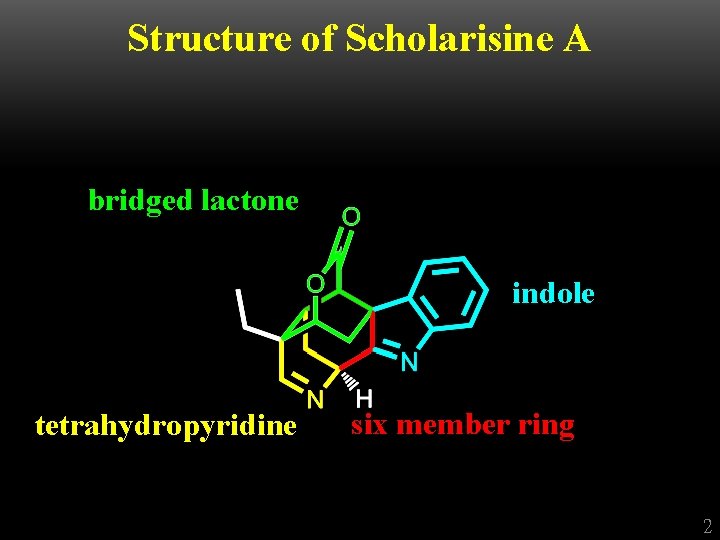
Structure of Scholarisine A bridged lactone indole tetrahydropyridine six member ring 2

Isolation of Scholarisine A • Scholarisine A (1), an akuammiline monoterpene indole alkaloid isolated from the leaves of Alstonia scholaris by Luo and co-workers. 3

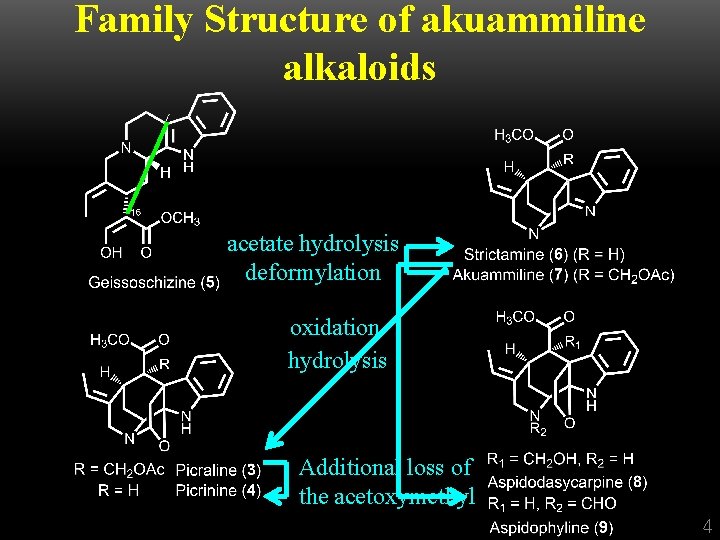
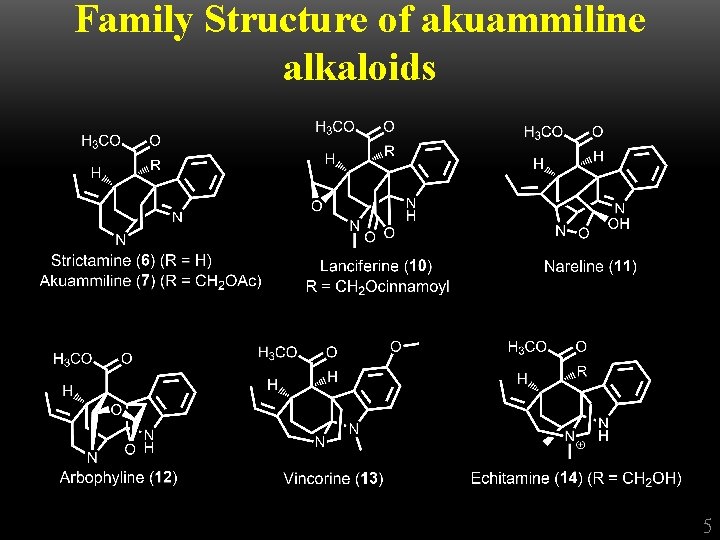
Family Structure of akuammiline alkaloids acetate hydrolysis deformylation oxidation hydrolysis Additional loss of the acetoxymethyl 4

Family Structure of akuammiline alkaloids 5

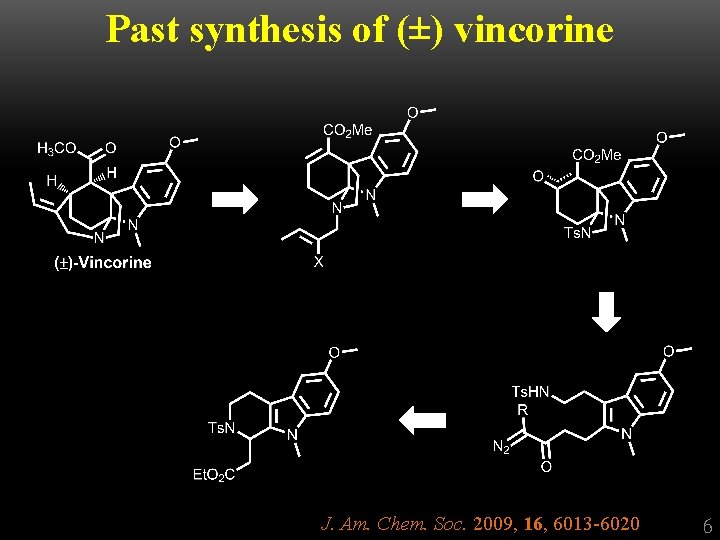
Past synthesis of (±) vincorine J. Am. Chem. Soc. 2009, 16, 6013 -6020 6

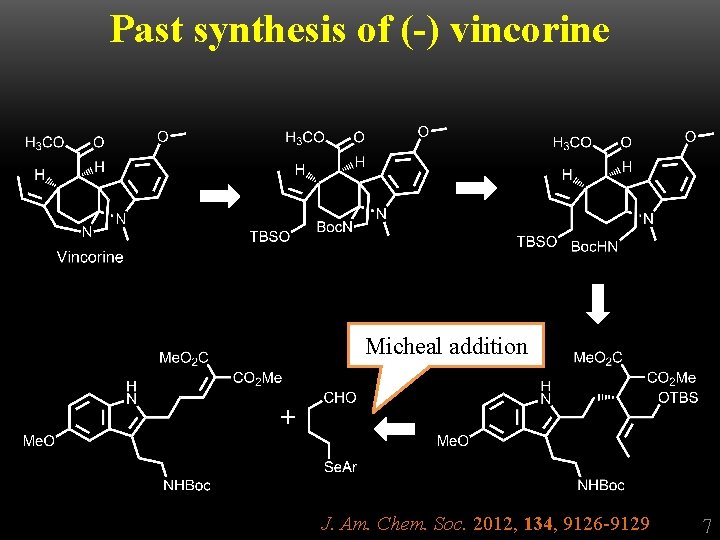
Past synthesis of (-) vincorine Micheal addition + J. Am. Chem. Soc. 2012, 134, 9126 -9129 7

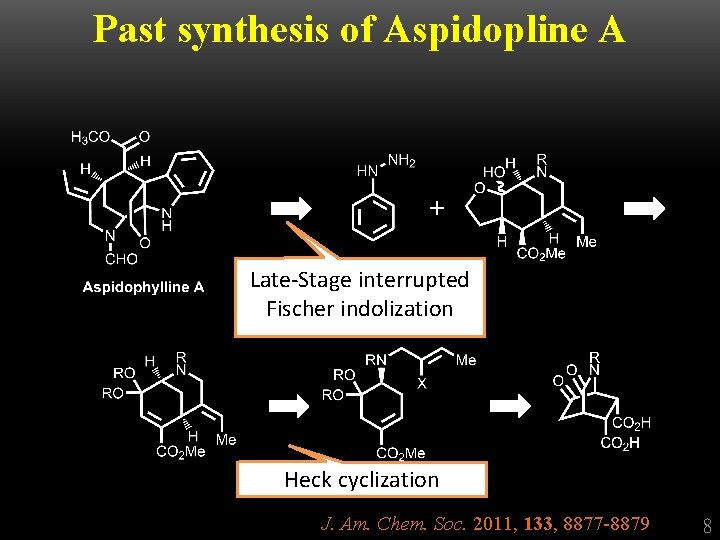
Past synthesis of Aspidopline A + Late-Stage interrupted Fischer indolization Heck cyclization J. Am. Chem. Soc. 2011, 133, 8877 -8879 8

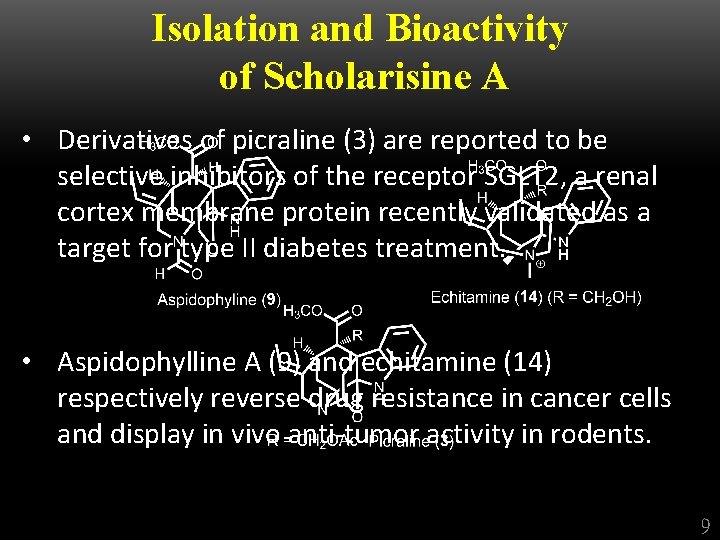
Isolation and Bioactivity of Scholarisine A • Derivatives of picraline (3) are reported to be selective inhibitors of the receptor SGLT 2, a renal cortex membrane protein recently validated as a target for type II diabetes treatment. • Aspidophylline A (9) and echitamine (14) respectively reverse drug resistance in cancer cells and display in vivo anti-tumor activity in rodents. 9

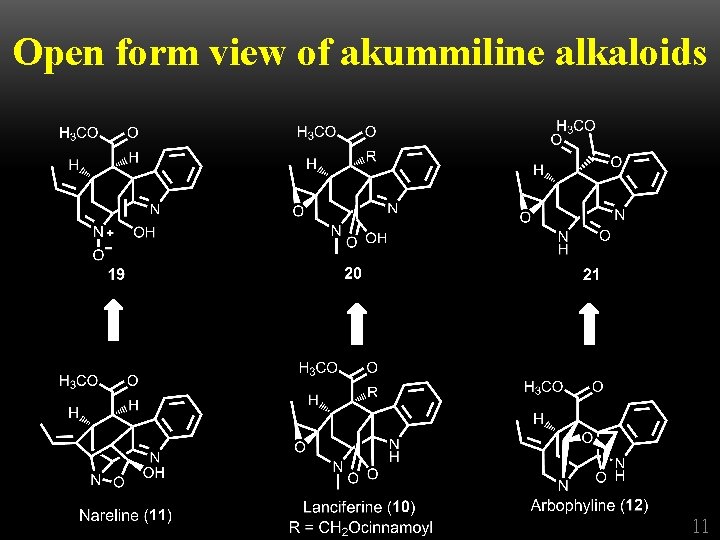
Open form view of akummiline alkaloids Tetrahedron, 1965, 21, 1717 -1734 10

Open form view of akummiline alkaloids 11

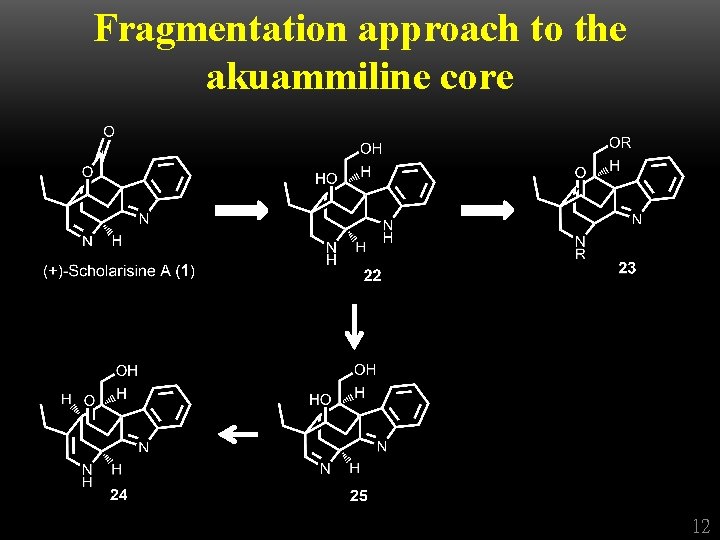
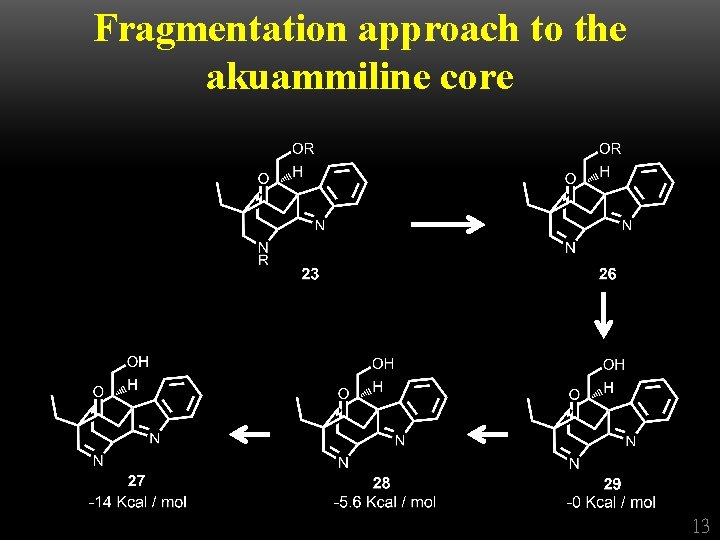
Fragmentation approach to the akuammiline core 12

Fragmentation approach to the akuammiline core 13

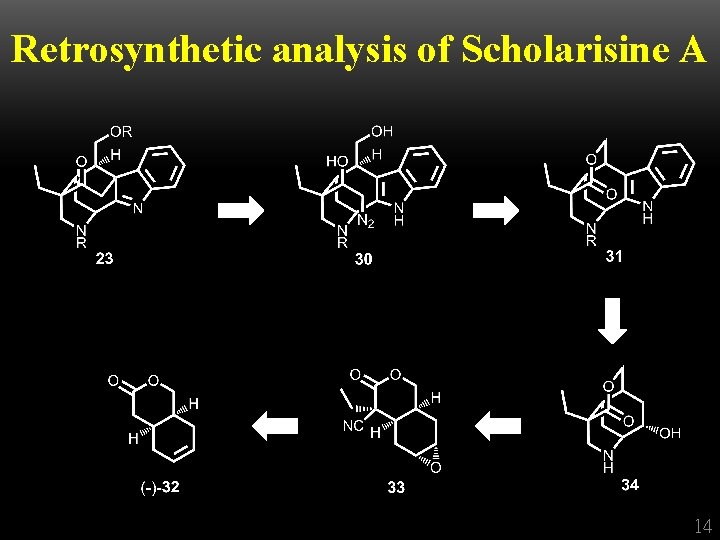
Retrosynthetic analysis of Scholarisine A 14

Preparation of lactone (-)-32 from anhydride 35 + 15

Synthesis of epoxide (-)-33 by m. CPBA + 3: 1 16

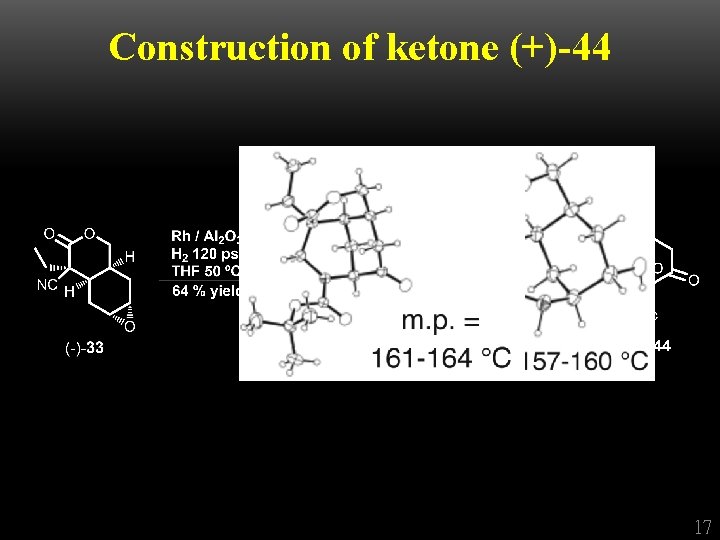
Construction of ketone (+)-44 17

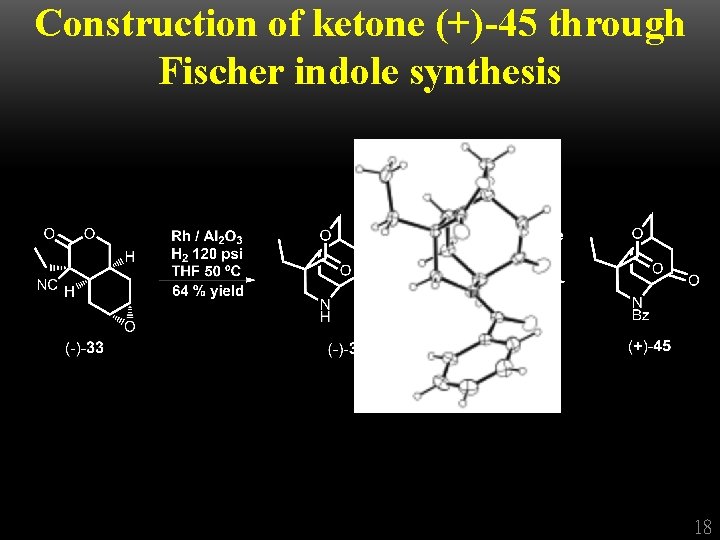
Construction of ketone (+)-45 through Fischer indole synthesis 18

Synthesis of phenylhydrazono 46 Fischer Indole Synthesis 19

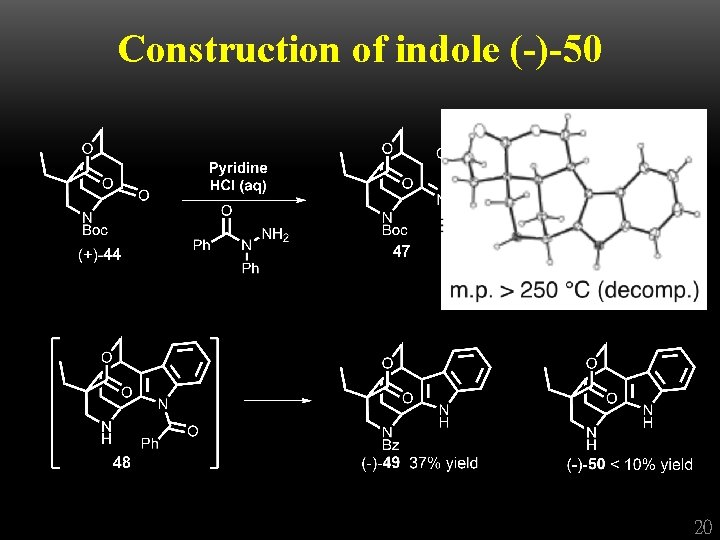
Construction of indole (-)-50 20

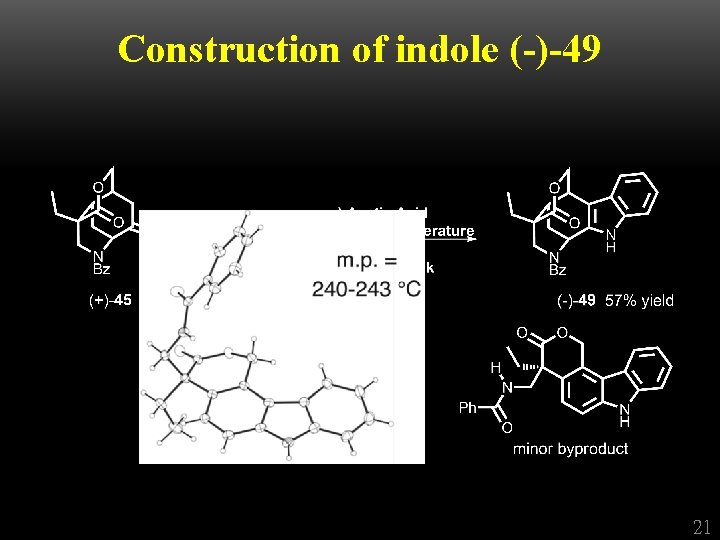
Construction of indole (-)-49 + 21

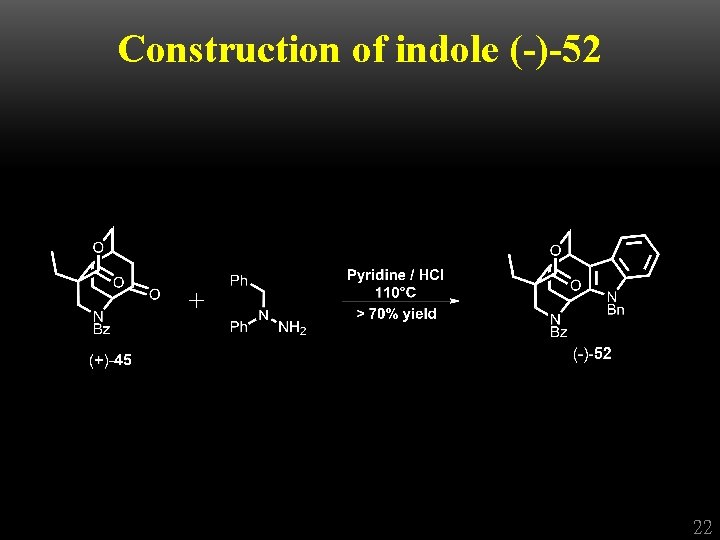
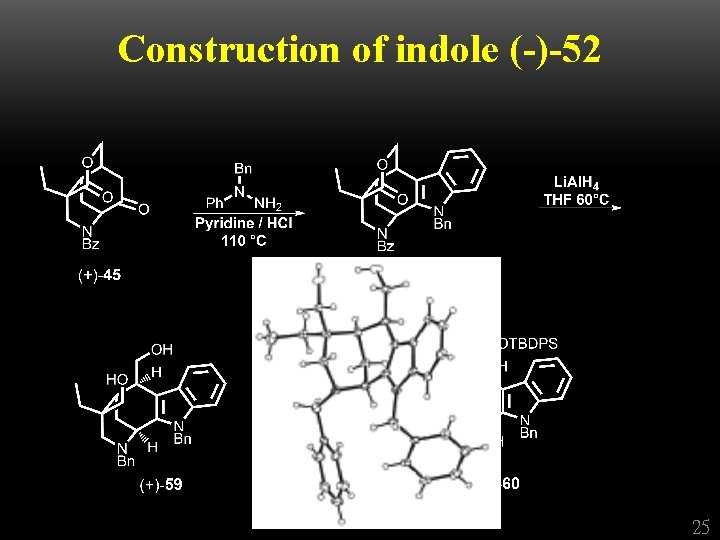
Construction of indole (-)-52 + 22

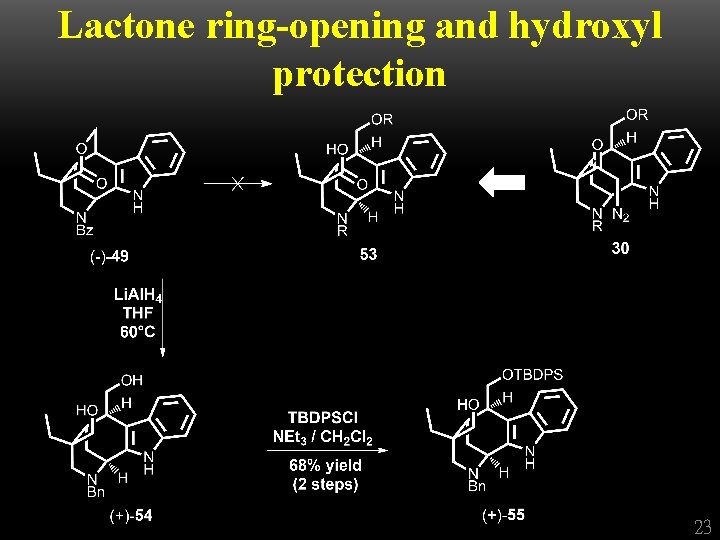
Lactone ring-opening and hydroxyl protection 23

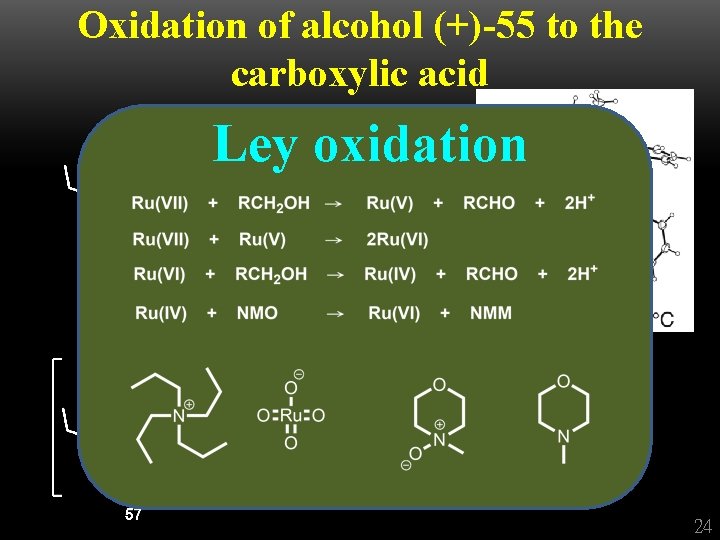
Oxidation of alcohol (+)-55 to the carboxylic acid Ley oxidation + 24

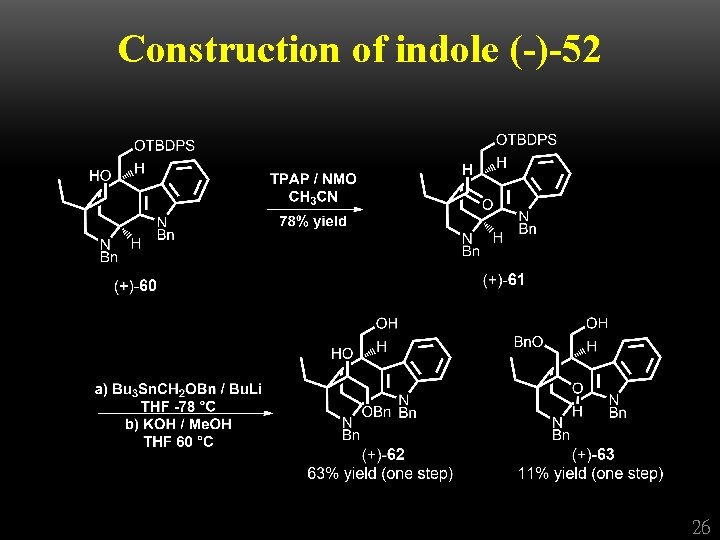
Construction of indole (-)-52 25

Construction of indole (-)-52 26

A new plan forward 27

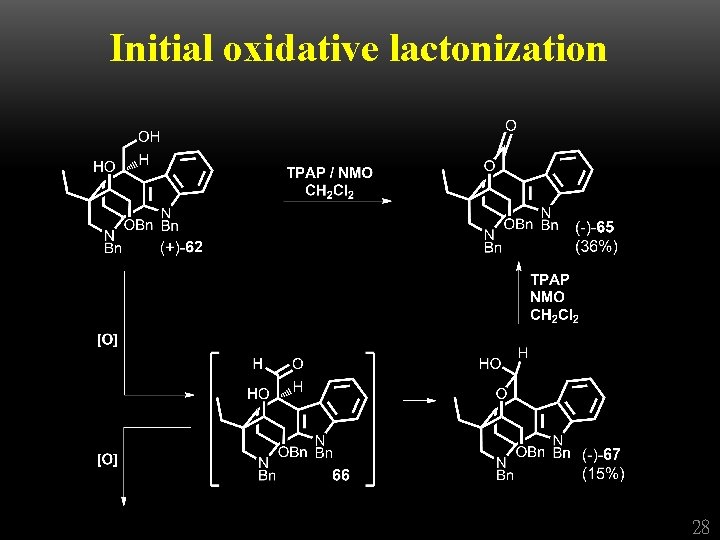
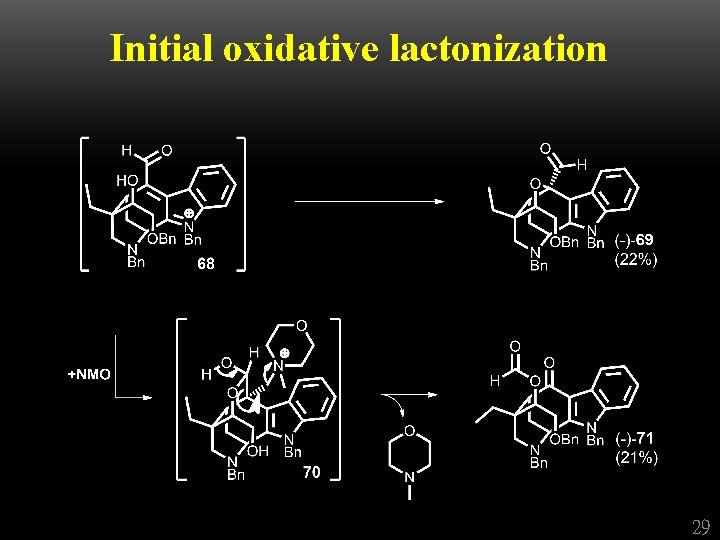
Initial oxidative lactonization 28

Initial oxidative lactonization 29

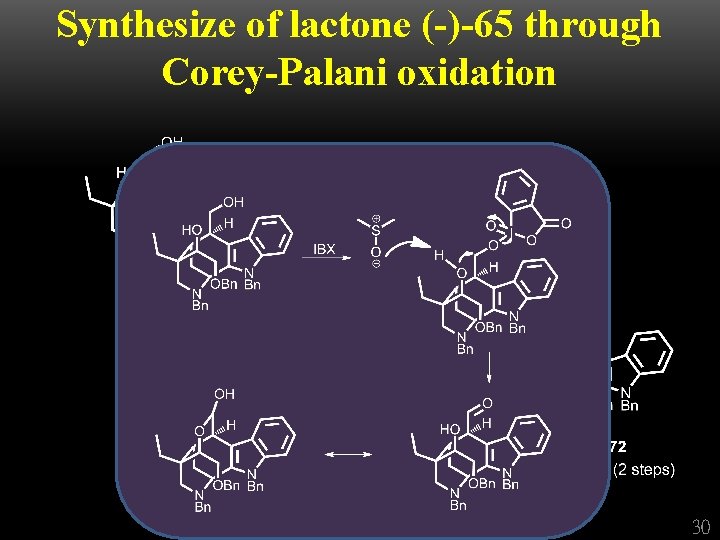
Synthesize of lactone (-)-65 through Corey-Palani oxidation 30

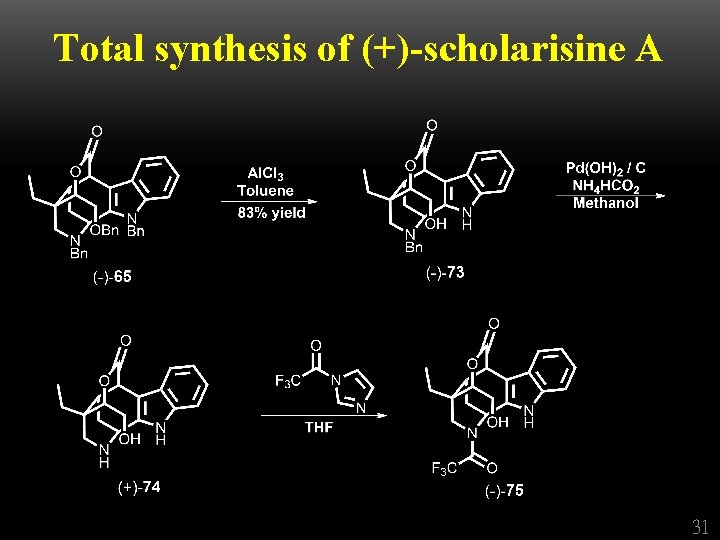
Total synthesis of (+)-scholarisine A 31

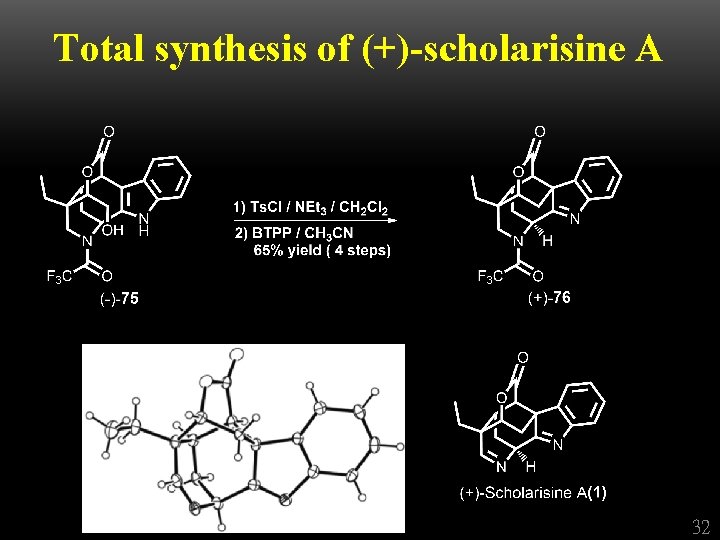
Total synthesis of (+)-scholarisine A 32

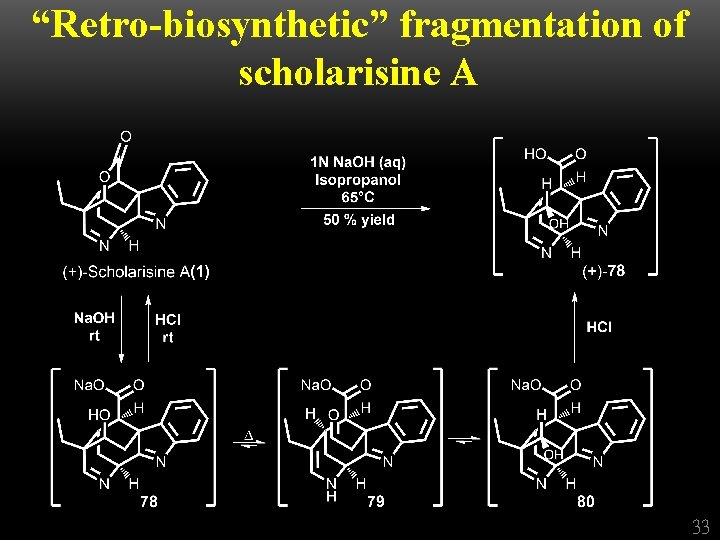
“Retro-biosynthetic” fragmentation of scholarisine A 33

Conclusion • The first total synthesis of (+)-scholarisine A (1) has been achieved, employing a longest linear reaction sequence of 25 steps from commercially available anhydride 35. • Key synthetic tactics include 1. a novel cyclization, comprising nitrile reduction coupled with concomitant addition of the resultant amine to an epoxide 2. a modified Fischer synthetic protocol 3. an oxidative lactonization of a diol in the presence of an indole ring 4. a late-stage cyclization to complete the caged ring scaffold of (+)scholarisine A (1). • A “retro-biosynthetic” fragmentation of totally synthetic (+)scholarisine A (1) has also been achieved. Studies are currently underway to exploit this fragmentation to gain access to related members of the akuammiline family. 34

Thanks for your attendance 35
 Akuammiline
Akuammiline Pyridine piperidine alkaloids
Pyridine piperidine alkaloids Norlupinane
Norlupinane Norlupinane
Norlupinane Uses of alkaloids in pharmacognosy
Uses of alkaloids in pharmacognosy Alkaloid ergoline
Alkaloid ergoline Alkaloid examples
Alkaloid examples Uses of alkaloids in pharmacognosy
Uses of alkaloids in pharmacognosy Alkaloids derived from tyrosine
Alkaloids derived from tyrosine Protoalkaloids
Protoalkaloids Purine alkaloids
Purine alkaloids Peomus boldus
Peomus boldus Purine alkaloids
Purine alkaloids Capsicum
Capsicum Solanacease
Solanacease Ergot alkaloids alpha blockers
Ergot alkaloids alpha blockers Ergot alkaloids alpha blockers
Ergot alkaloids alpha blockers Formula de roe
Formula de roe Total revenues minus total costs equals
Total revenues minus total costs equals Total revenues minus total costs equals
Total revenues minus total costs equals Total revenues minus total costs equals
Total revenues minus total costs equals Total revenue minus total expenses
Total revenue minus total expenses Total query trong access
Total query trong access Terminal access controller access-control system
Terminal access controller access-control system Terminal access controller access-control system
Terminal access controller access-control system Skyward family access psl
Skyward family access psl Classroom google calendar
Classroom google calendar Carroll isd naviance
Carroll isd naviance Skyward canyons
Skyward canyons Sksd skyward
Sksd skyward Seating chart in skyward
Seating chart in skyward Ccisd.skyward
Ccisd.skyward Skyward hrp
Skyward hrp Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể