Access to Antimalarial Medicines Dr Clive O Ondari














- Slides: 20

Access to Antimalarial Medicines Dr Clive O Ondari Essential Drugs and Medicines Policy Dept & Roll Back Malaria Department World Health Organization October 2003

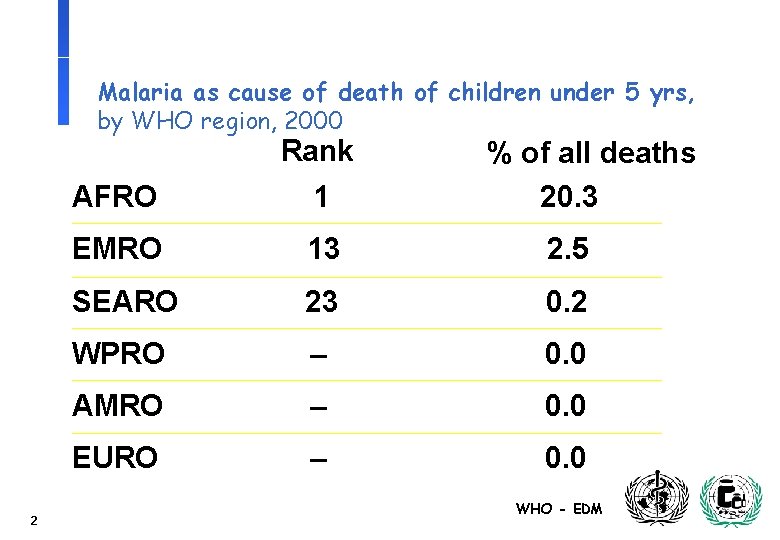
Malaria as cause of death of children under 5 yrs, by WHO region, 2000 Rank 2 % of all deaths 20. 3 AFRO 1 EMRO 13 2. 5 SEARO 23 0. 2 WPRO – 0. 0 AMRO – 0. 0 EURO – 0. 0 WHO - EDM

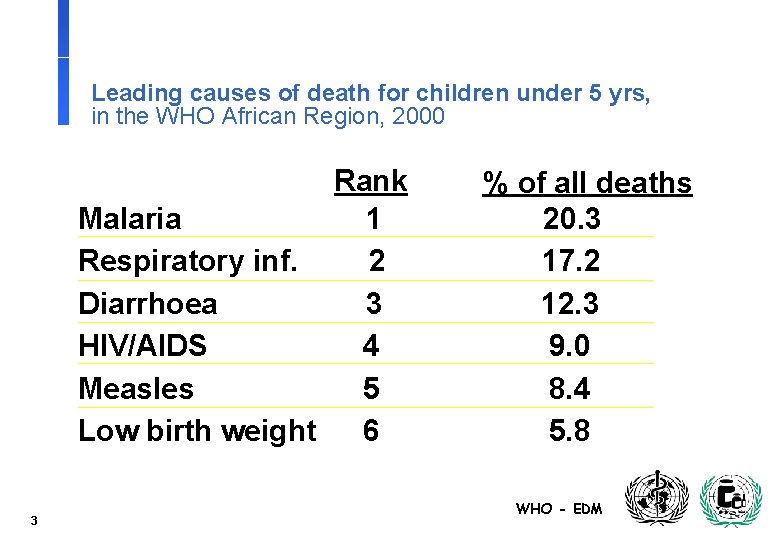
Leading causes of death for children under 5 yrs, in the WHO African Region, 2000 Rank Malaria 1 Respiratory inf. 2 Diarrhoea 3 HIV/AIDS 4 Measles 5 Low birth weight 6 3 % of all deaths 20. 3 17. 2 12. 3 9. 0 8. 4 5. 8 WHO - EDM

Current Situation: n n n 4 Quality of antimalarial drugs has been declining. The efficacy of (affordable) antimalarial drugs has been declining and high cost of replacement options. Over 50% of the population does not have regular access to most vital essential drugs. 60 -90% of the population seek initial treatment from unqualified sources, i. e. street vendors, kiosks. Supply of drugs is often inefficient and unreliable. Use of ineffective drugs leads to inadequate treatment and leads to drug resistance. WHO - EDM

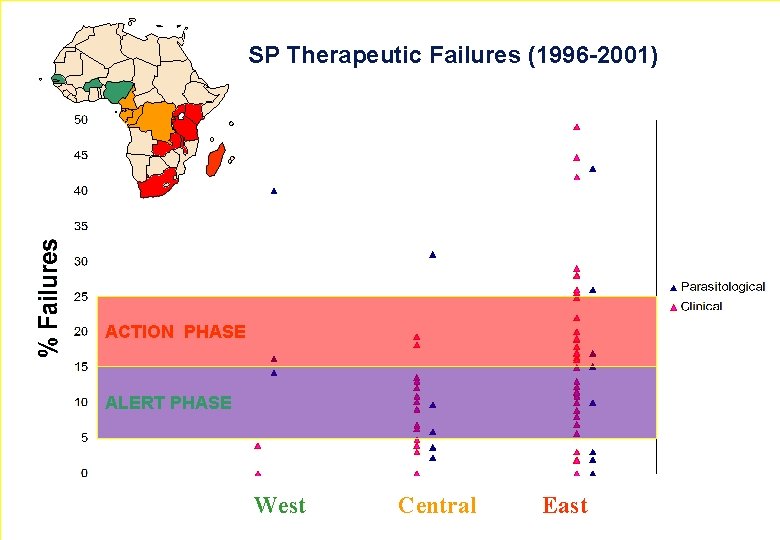
SP Therapeutic Failures (1996 -2001) ACTION PHASE ALERT PHASE 5 West Central East WHO - EDM

Problems of resistance to antimalarial drugs n n n 6 Era of availability of cheap and effective drugs has come to an end Both CQ & SP cost <US$0. 2/adult treatment course, but resistance to CQ widespread, and to SP increasing Alternatives for multi-drug resistant falciparum malaria are x 10 expensive WHO - EDM

Factors leading to development of resistance n n n 7 Lack of guidelines/poor drug treatment policies Irrational prescribing Irrational drug use Drug concentration “tail” Liberalized, uncontrolled drug market leading to poor quality products circulating in international and domestic markets WHO - EDM

The Abuja Declaration, African Summit on Roll Back Malaria (Abuja, Nigeria), April 2000 Call upon all member states to: 1. “Make treatment of malaria available as peripherally as possible including home treatment” 2. “Make appropriate treatment available and accessible to the poorest groups in the community” Pledge to: 1. “Reduce or waive taxes and tariffs for …antimalarial drugs” 2. “Explore and develop traditional medicine in the area of malaria control” 8 WHO - EDM

Developing and promoting interventions to improve access to good quality antimalarial drugs · Rational selection and use WHO will continue to work with member states to ensure that: 1) 2) 3) 4) 9 Evidence that is accumulated in the clinical practice is used to develop and update malaria treatment guidelines, Treatment guidelines form the basis for the development of essential drugs list Essential drugs list guide the procurement, distribution and use of antimalarial drugs New antimalarial drugs, including novel combinations, are evaluated adequately and registered in a timely manner. WHO - EDM

Rational Antimalarial Drug Selection 10 WHO - EDM

Recommended combination antimalarials: n n 11 Artesunate + amodiaquine Artesunate + sulfadoxine-pyrimethamine Artesunate + mefloquine Artemether + lumefantrine WHO - EDM

Average cost per adult treatment (US$) Cost implications of combination therapy 12 WHO - EDM

Global negotiation to reduce price of new antimalarials 13 n Expert consensus that co-formulated artemisinin combination best option for use where multi-resistant malaria n Artemether/lumefantrine (Coartem) is only medicine of this type currently licensed n Novartis has agreed to provide drug at cost through WHO for 10 years – US$ 2. 40 for adult treatment n Countries to request, direct or through NGOs WHO - EDM

Developing and promoting interventions to improve access to good quality antimalarial drugs · Sustainable financing Efforts are directed towards: 1) Developing guidelines on sustainable financing 2) Dissemination and promotion of interagency guidelines on drug donation 3) Advocacy for increased budget allocation for essential drugs 14 WHO - EDM

Pilot Project on Quality of Antimalarial Products n Project Design : ä ä 15 Country selection criteria: “spot light countries” in AFRO and EMRO Evaluation of the most widely used antimalarials products in the regions Sampling from various levels of the distribution chain (household, private sector pharmacy, peripheral health units, district hospital, teaching/referral hospital and district and/or central medical store Evaluation of samples on a rapid assessment kit (Mini-lab) and central laboratory (pharmacopeal tests) in CENQAM, South Africa WHO - EDM

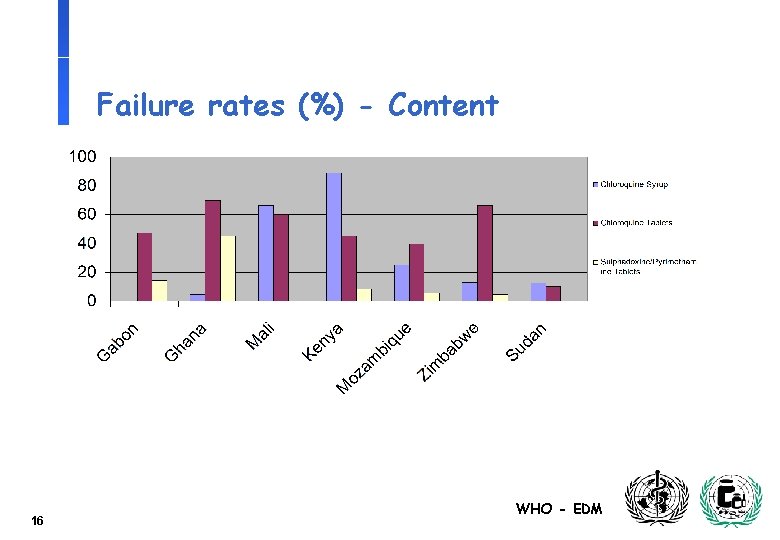
Failure rates (%) - Content 16 WHO - EDM

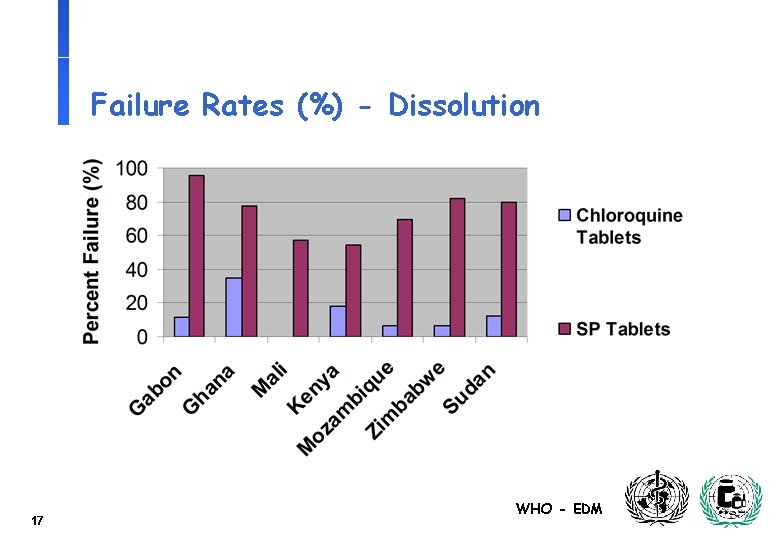
Failure Rates (%) - Dissolution 17 WHO - EDM

Pre-qualification of ACT Manufacturers and Products n Objectives: ä To accelerate sustained access to, and use of, good quality ACTs ä To ensure that adequate and effective treatment reaches significantly greater numbers of people in need ä To assist/support the implementation of ACTs in ways that respond to the specific needs and requests of individual countries ä 18 To support drug regulatory agencies in regulating ACTs WHO - EDM

RBM/EDM Planned Activities - Biennium 20042005 n Rational Selection and Use ä ä n Affordable Prices for Antimalarial Drugs ä ä ä 19 Collect and collate efficacy and safety data on new AM Develop training materials for new AM Assist in the dissemination of training materials Provide assistance in training activities Collect information on pricing/prices of antimalarials Assist countries in the procurement of antimalarials Collect data on domestic manufacturing - capacity etc WHO - EDM

RBM/EDM Planned Activities - Biennium 20042005 n Providing assistance in ensuring sustainable financing ä ä Develop a database on national budgets/expenditures on AM Develop a database on the availability of AM (esp. ACTs) n Strengthening drug supply, quality assurance and regulatory systems ä ä 20 Develop guidelines for registration of new antimalarials Assist countries to evaluate quality and monitor inspection activities (including pre-qualification of sources of products) Provide support to training in the areas of pharmaceutical inspection (GMP) - manufacturing and distribution channels Initiate the development of monographs for new AM WHO - EDM
 Clive ondari
Clive ondari Medicines information centre
Medicines information centre Cqc medicines management
Cqc medicines management European directorate for the quality of medicines
European directorate for the quality of medicines Ggc medicines
Ggc medicines Ectoparasiticides veterinary medicines
Ectoparasiticides veterinary medicines Refrigerant management program
Refrigerant management program Medicinescomplete martindale
Medicinescomplete martindale What legislation helped solve dangerous food and medicines
What legislation helped solve dangerous food and medicines Federal agency for medicines and health products
Federal agency for medicines and health products Veterinary medicines directorate
Veterinary medicines directorate European medicines agency
European medicines agency Complete floor stock system of drug distribution
Complete floor stock system of drug distribution Pharmac pharmaceutical schedule
Pharmac pharmaceutical schedule Nhs dictionary of medicines and devices
Nhs dictionary of medicines and devices Chapter 19 vocabulary glencoe health
Chapter 19 vocabulary glencoe health Medicines complete
Medicines complete Herbal medicine approved by doh
Herbal medicine approved by doh National medicines policy
National medicines policy Medicines learning portal
Medicines learning portal Staff of marvelous medicines
Staff of marvelous medicines