Access Closure Devices A Changing Paradigm Mark A









- Slides: 42

Access Closure Devices: A Changing Paradigm Mark A. Turco, MD, FACC, FSCAI Director, Center for Cardiac and Vascular Research Washington Adventist Hospital Takoma Park, Maryland Assistant Professor of Medicine Uniformed Services University of the Health Sciences Bethesda, Maryland

DISCLOSURES Mark A. Turco, MD Honoraria – Boston Scientific, Medtronic Cardio. Vascular, Inc. , Abbott Vascular Grants/Contracted Research – Boston Scientific, Medtronic Cardio. Vascular, Inc. , Abbott Vascular

Limitations of manual compression Ø Delayed ambulation Ø Patient dissatisfaction/discomfort Ø Time and personnel intensive ØVascular complications in anticoagulated pts still occur after successful hemostasis obtained by manual compression

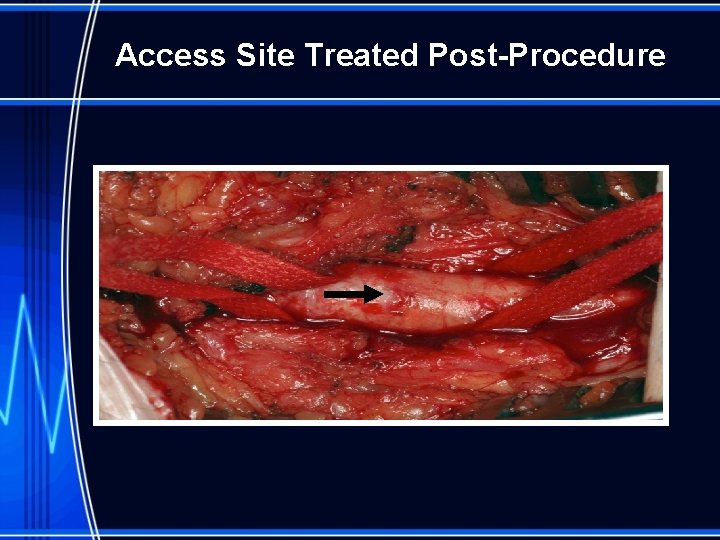
Access Site Treated Post-Procedure

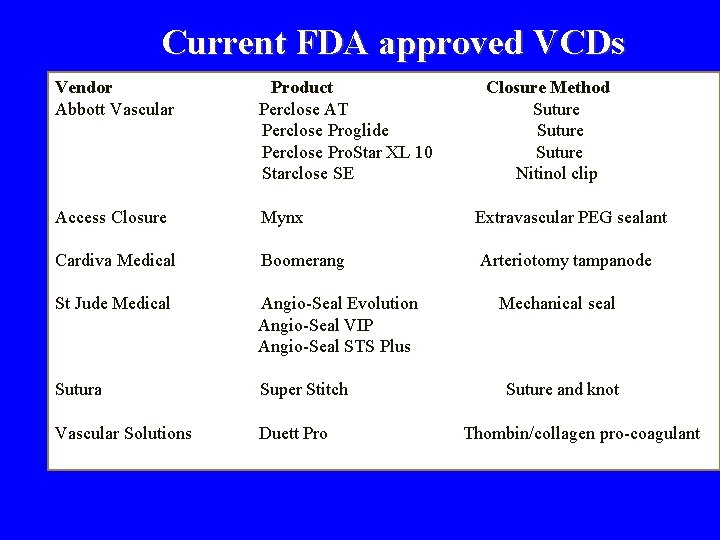
Current FDA approved VCDs Vendor Abbott Vascular Product Perclose AT Perclose Proglide Perclose Pro. Star XL 10 Starclose SE Closure Method Suture Nitinol clip Access Closure Mynx Extravascular PEG sealant Cardiva Medical Boomerang Arteriotomy tampanode St Jude Medical Angio-Seal Evolution Angio-Seal VIP Angio-Seal STS Plus Sutura Super Stitch Vascular Solutions Duett Pro Mechanical seal Suture and knot Thombin/collagen pro-coagulant

Closure Technology ACTIVE VS PASSIVE • ACTIVE: Mechanical approximators are active ie. Angio-Seal, Perclose, Starclose • PASSIVE: Cardiva Catalyst, Mynx • INTRALUMINAL vs EXTRALUMINAL ie. Mynx and Star. Close are Extraluminal

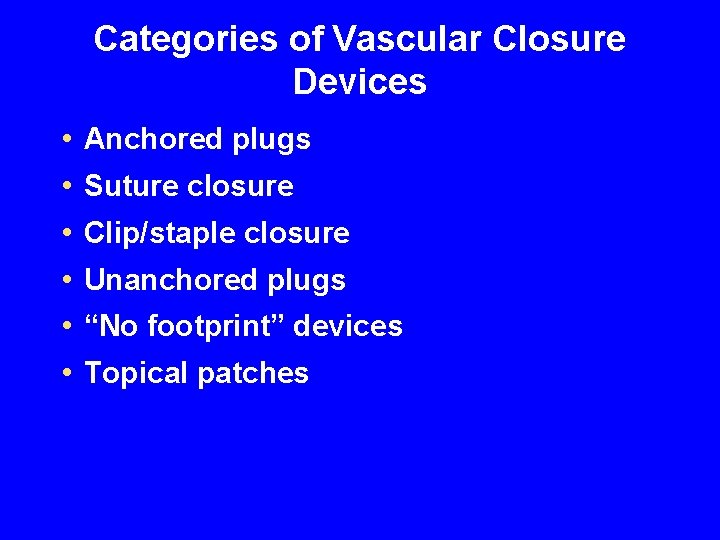
Categories of Vascular Closure Devices • • • Anchored plugs Suture closure Clip/staple closure Unanchored plugs “No footprint” devices Topical patches

Closure Begins with Access • Decision to use ¡ made before access • Closure technique ¡ in place during the case itself ¡ rather than deployed in entirety after

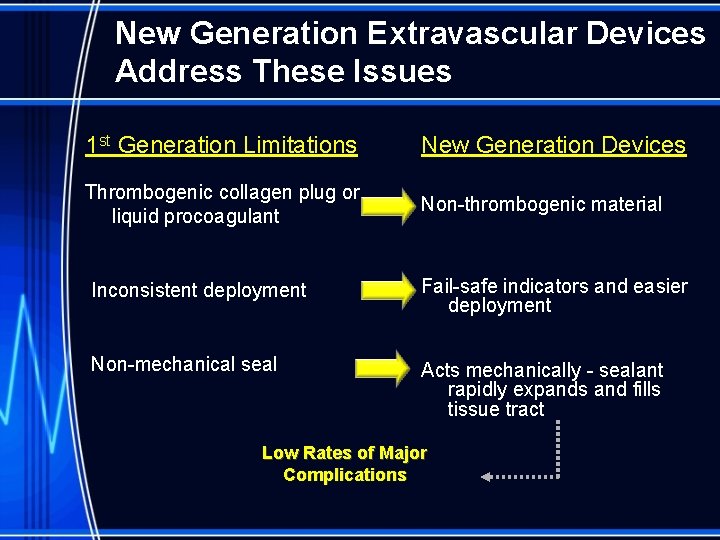
New Generation Extravascular Devices Address These Issues 1 st Generation Limitations New Generation Devices Thrombogenic collagen plug or liquid procoagulant Non-thrombogenic material Inconsistent deployment Fail-safe indicators and easier deployment Non-mechanical seal Acts mechanically - sealant rapidly expands and fills tissue tract Low Rates of Major Complications

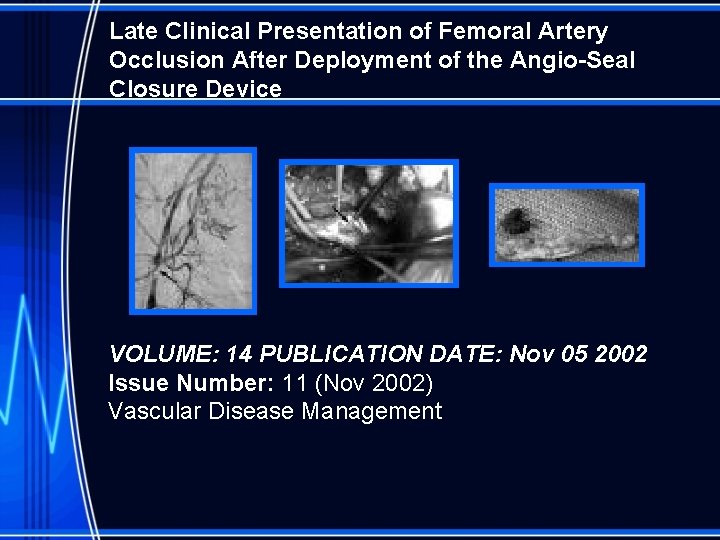
Late Clinical Presentation of Femoral Artery Occlusion After Deployment of the Angio-Seal Closure Device VOLUME: 14 PUBLICATION DATE: Nov 05 2002 Issue Number: 11 (Nov 2002) Vascular Disease Management

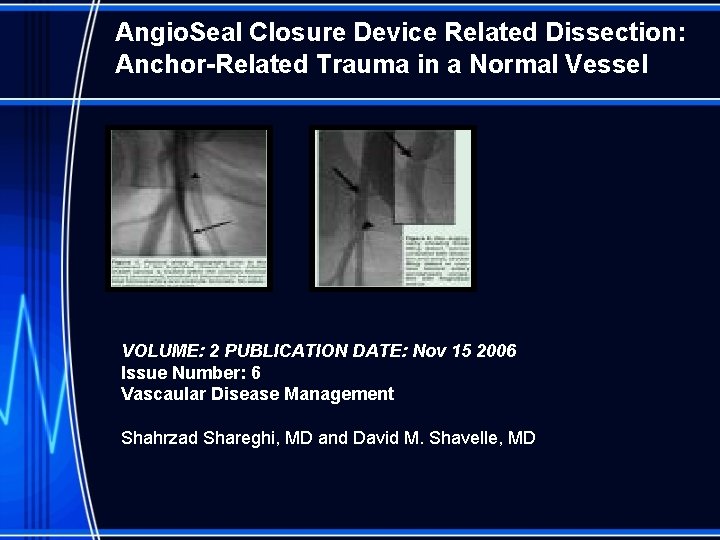
Angio. Seal Closure Device Related Dissection: Anchor-Related Trauma in a Normal Vessel VOLUME: 2 PUBLICATION DATE: Nov 15 2006 Issue Number: 6 Vascaular Disease Management Shahrzad Shareghi, MD and David M. Shavelle, MD

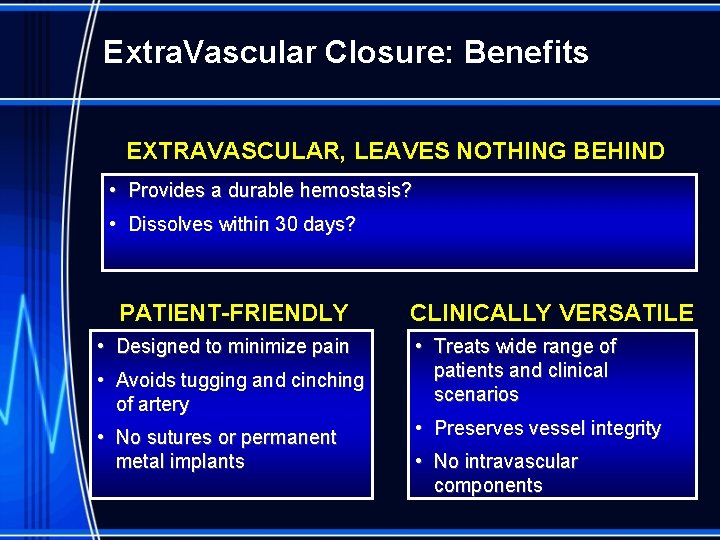
Benefits of Extravascular Closure § No risk of flow obstruction from intraluminal components § Lower risk of catastrophic complications – no intravascular components that can detach and lodge distally. § Clinical versatility – fewer anatomical constraints that typically limit intra-arterial VCD use § Lack of inflammation and scar tissue from intraarterial components

Extra. Vascular Closure: Benefits EXTRAVASCULAR, LEAVES NOTHING BEHIND • Provides a durable hemostasis? • Dissolves within 30 days? PATIENT-FRIENDLY • Designed to minimize pain • Avoids tugging and cinching of artery • No sutures or permanent metal implants CLINICALLY VERSATILE • Treats wide range of patients and clinical scenarios • Preserves vessel integrity • No intravascular components

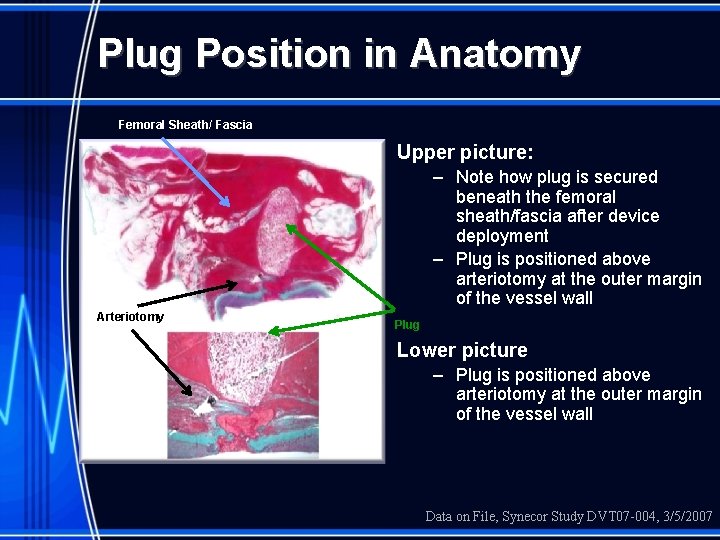
Plug Position in Anatomy Femoral Sheath/ Fascia Upper picture: – Note how plug is secured beneath the femoral sheath/fascia after device deployment – Plug is positioned above arteriotomy at the outer margin of the vessel wall Arteriotomy Plug Lower picture – Plug is positioned above arteriotomy at the outer margin of the vessel wall Data on File, Synecor Study DVT 07 -004, 3/5/2007

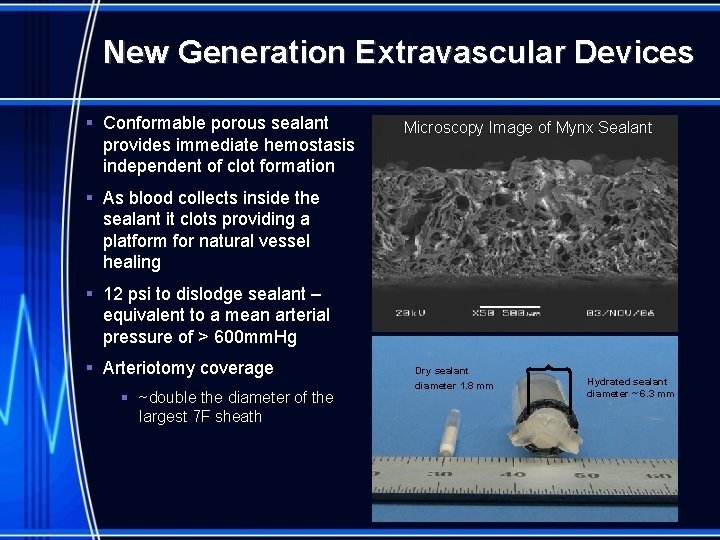
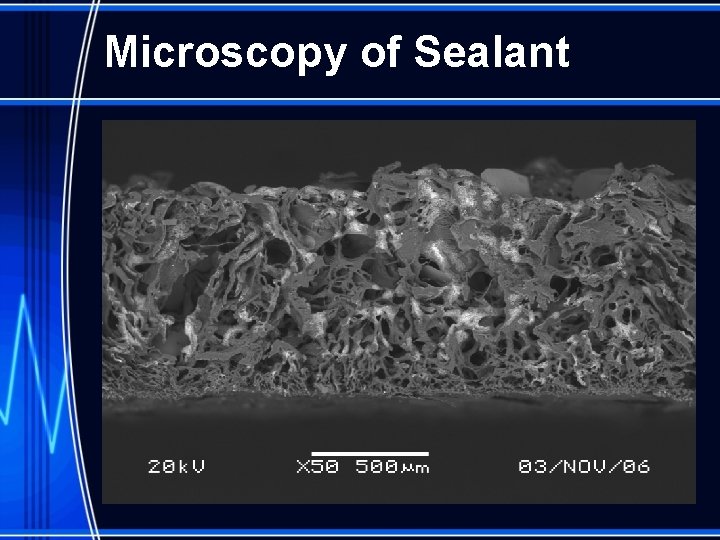
New Generation Extravascular Devices § Conformable porous sealant provides immediate hemostasis independent of clot formation Microscopy Image of Mynx Sealant § As blood collects inside the sealant it clots providing a platform for natural vessel healing § 12 psi to dislodge sealant – equivalent to a mean arterial pressure of > 600 mm. Hg § Arteriotomy coverage § ~double the diameter of the largest 7 F sheath Dry sealant diameter 1. 8 mm Hydrated sealant diameter ~ 6. 3 mm

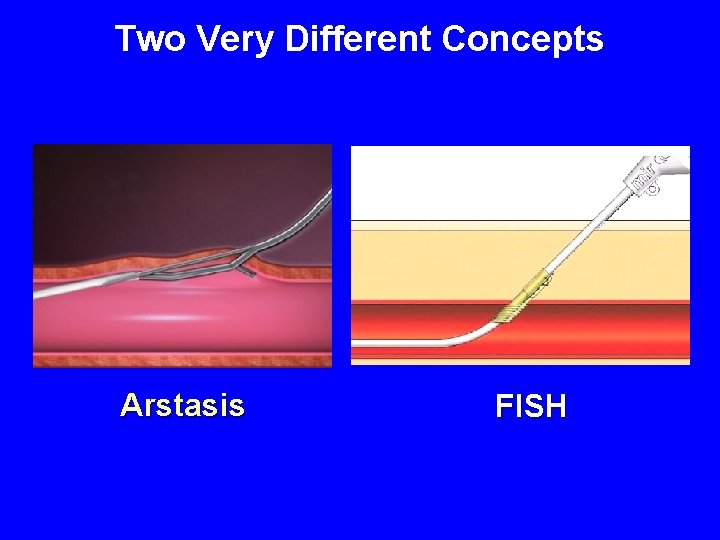
New Technology • MYNX • FISH • ARSTASIS • BOOMERANG

® The Mynx Vascular Closure Device Passive and Extraluminal

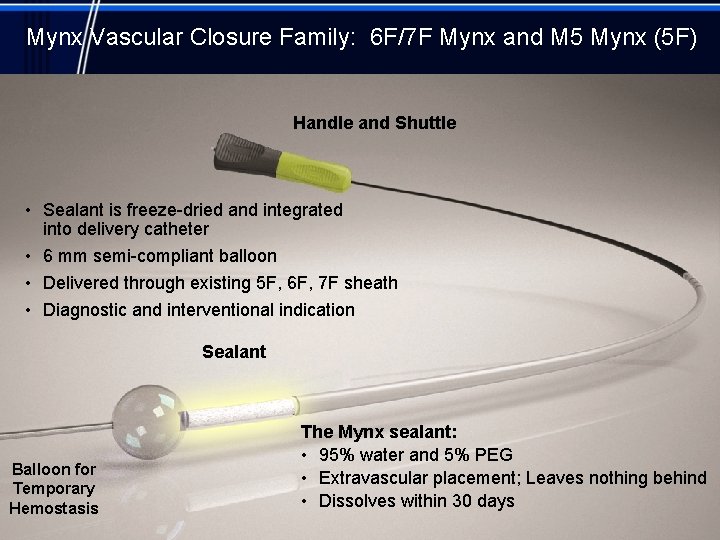
Mynx Vascular Closure Family: 6 F/7 F Mynx and M 5 Mynx (5 F) Handle and Shuttle • Sealant is freeze-dried and integrated into delivery catheter • 6 mm semi-compliant balloon • Delivered through existing 5 F, 6 F, 7 F sheath • Diagnostic and interventional indication Sealant Balloon for Temporary Hemostasis The Mynx sealant: • 95% water and 5% PEG • Extravascular placement; Leaves nothing behind • Dissolves within 30 days

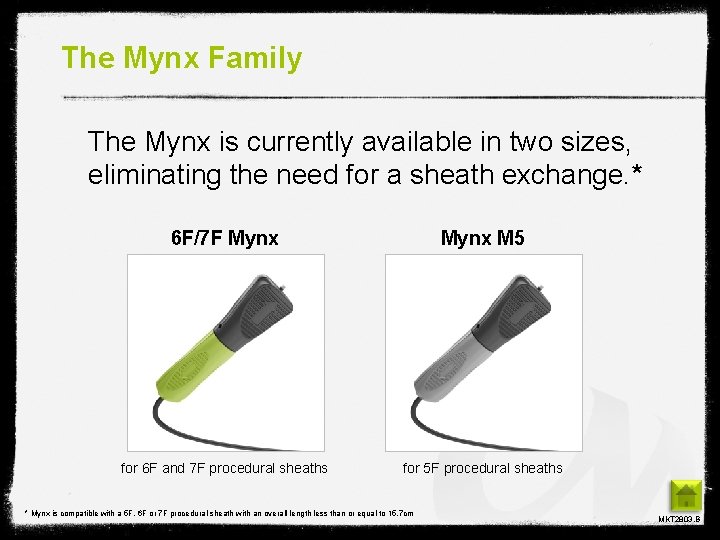
The Mynx Family The Mynx is currently available in two sizes, eliminating the need for a sheath exchange. * 6 F/7 F Mynx M 5 for 6 F and 7 F procedural sheaths for 5 F procedural sheaths * Mynx is compatible with a 5 F, 6 F or 7 F procedural sheath with an overall length less than or equal to 15. 7 cm MKT 2803. B

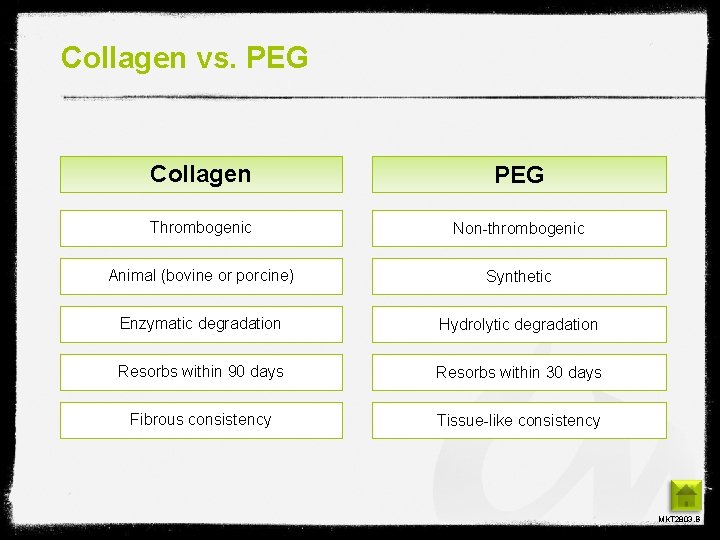
Collagen vs. PEG Collagen PEG Thrombogenic Non-thrombogenic Animal (bovine or porcine) Synthetic Enzymatic degradation Hydrolytic degradation Resorbs within 90 days Resorbs within 30 days Fibrous consistency Tissue-like consistency MKT 2803. B

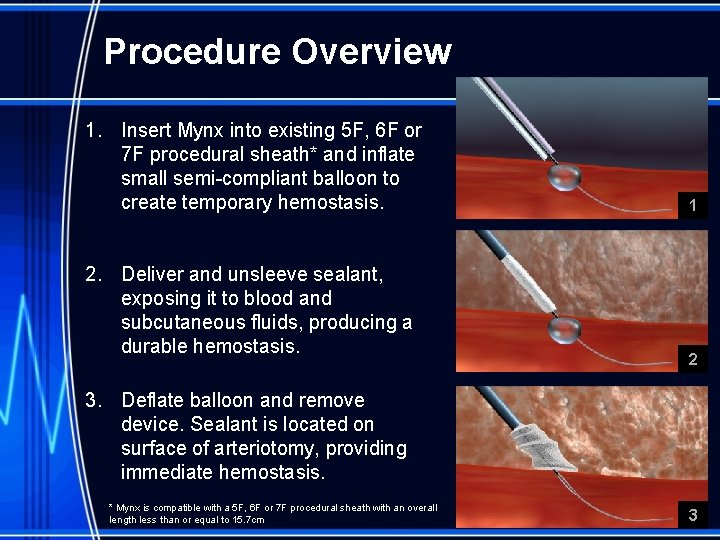
Procedure Overview 1. Insert Mynx into existing 5 F, 6 F or 7 F procedural sheath* and inflate small semi-compliant balloon to create temporary hemostasis. 2. Deliver and unsleeve sealant, exposing it to blood and subcutaneous fluids, producing a durable hemostasis. 1 2 3. Deflate balloon and remove device. Sealant is located on surface of arteriotomy, providing immediate hemostasis. * Mynx is compatible with a 5 F, 6 F or 7 F procedural sheath with an overall length less than or equal to 15. 7 cm 3

Microscopy of Sealant

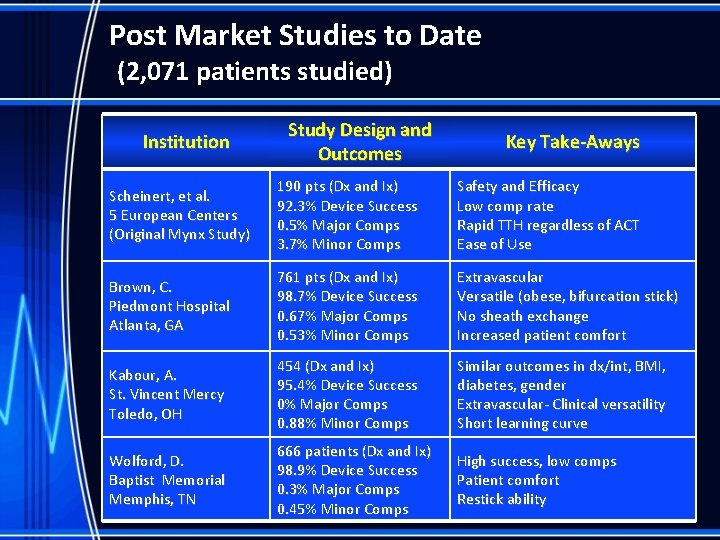
Post Market Studies to Date (2, 071 patients studied) Institution Study Design and Outcomes Key Take-Aways Scheinert, et al. 5 European Centers (Original Mynx Study) 190 pts (Dx and Ix) 92. 3% Device Success 0. 5% Major Comps 3. 7% Minor Comps Safety and Efficacy Low comp rate Rapid TTH regardless of ACT Ease of Use Brown, C. Piedmont Hospital Atlanta, GA 761 pts (Dx and Ix) 98. 7% Device Success 0. 67% Major Comps 0. 53% Minor Comps Extravascular Versatile (obese, bifurcation stick) No sheath exchange Increased patient comfort Kabour, A. St. Vincent Mercy Toledo, OH 454 (Dx and Ix) 95. 4% Device Success 0% Major Comps 0. 88% Minor Comps Similar outcomes in dx/int, BMI, diabetes, gender Extravascular- Clinical versatility Short learning curve Wolford, D. Baptist Memorial Memphis, TN 666 patients (Dx and Ix) 98. 9% Device Success 0. 3% Major Comps 0. 45% Minor Comps High success, low comps Patient comfort Restick ability

Two Very Different Concepts Arstasis FISH

FISH

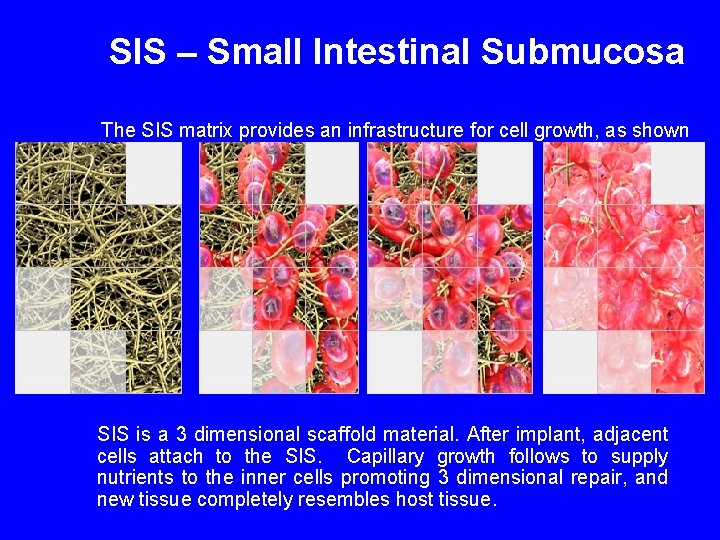
SIS – Small Intestinal Submucosa The SIS matrix provides an infrastructure for cell growth, as shown below: SIS is a 3 dimensional scaffold material. After implant, adjacent cells attach to the SIS. Capillary growth follows to supply nutrients to the inner cells promoting 3 dimensional repair, and new tissue completely resembles host tissue.

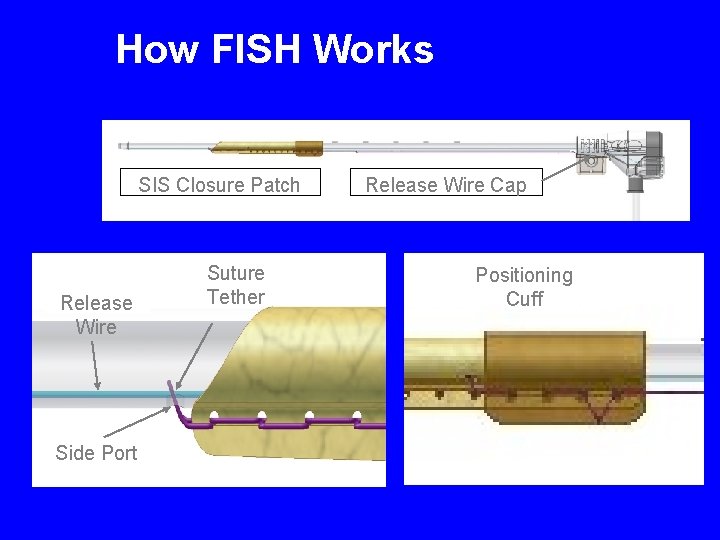
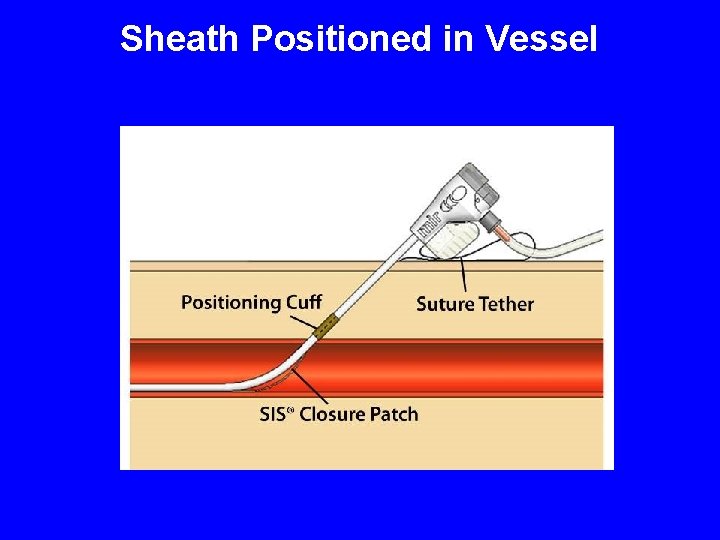
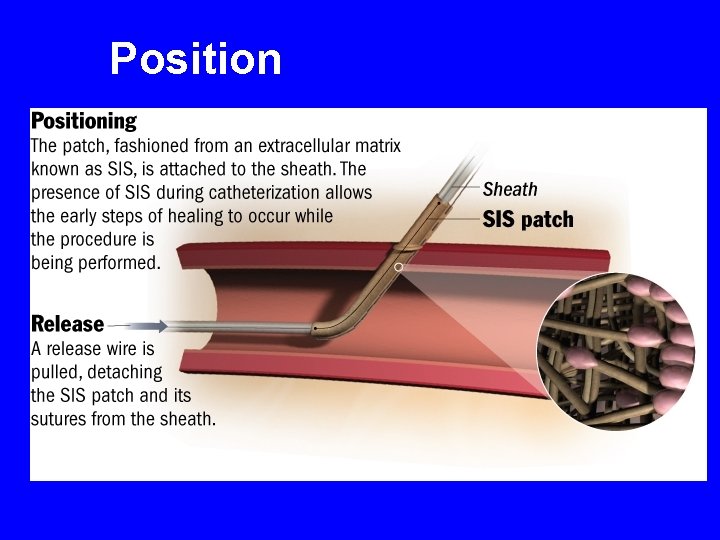
How FISH Works SIS Closure Patch Release Wire Side Port Suture Tether Release Wire Cap Positioning Cuff

Sheath Positioned in Vessel

Position

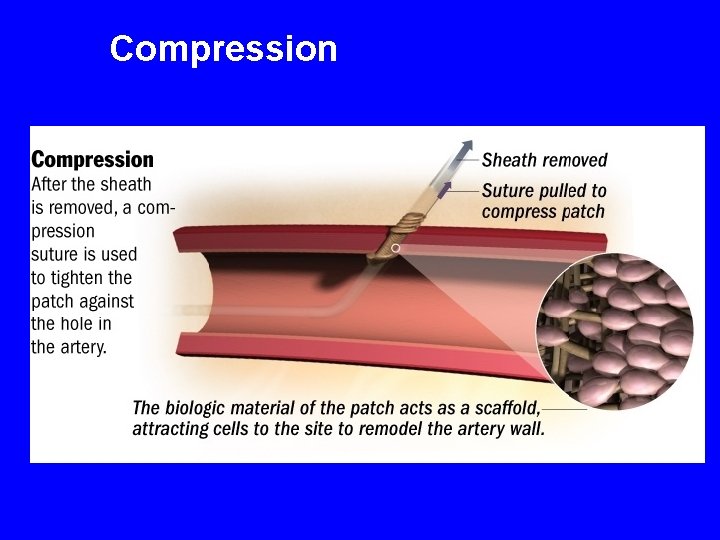
Compression

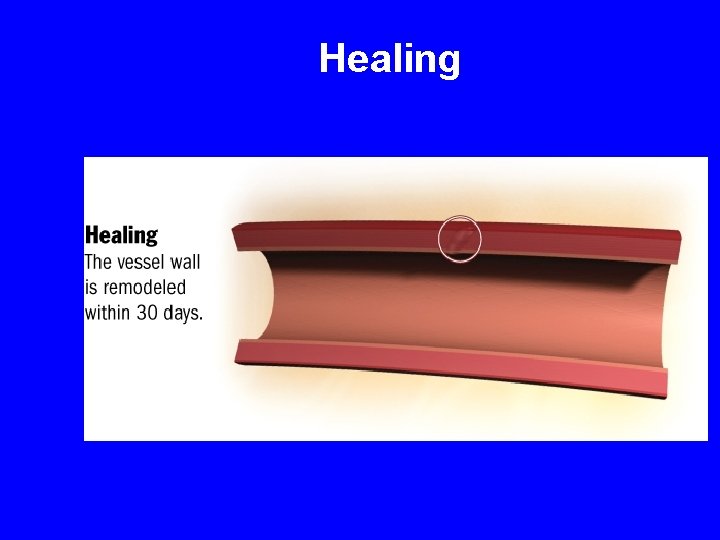
Healing


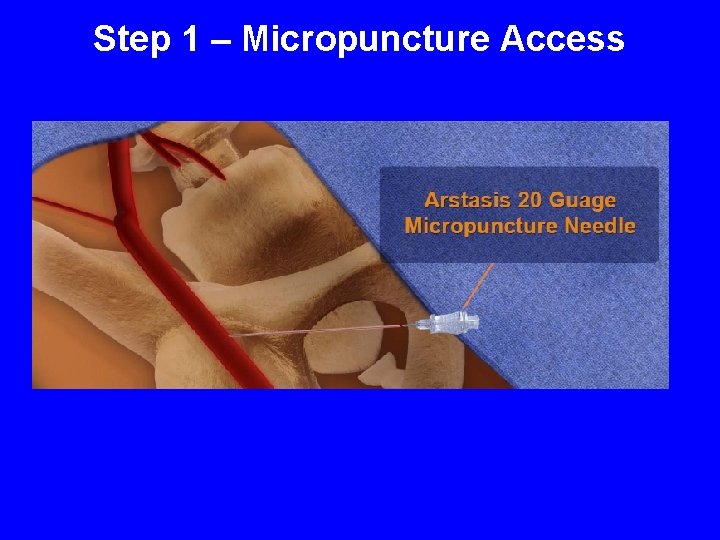
Step 1 – Micropuncture Access

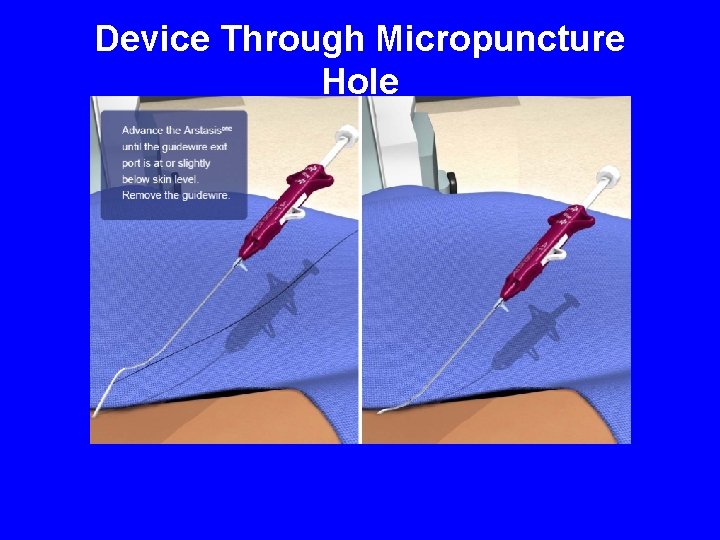
Device Through Micropuncture Hole

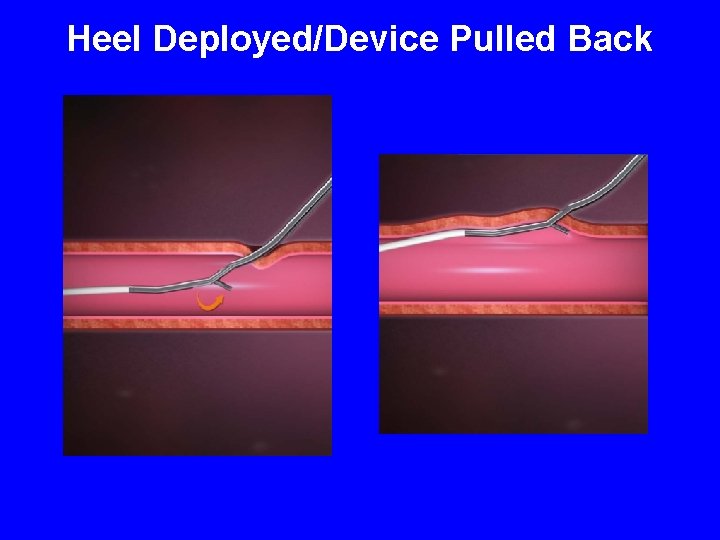
Heel Deployed/Device Pulled Back

Sheath Access Path Created Needle deployed at shallow angle across vessel wall Wire then advanced through needle

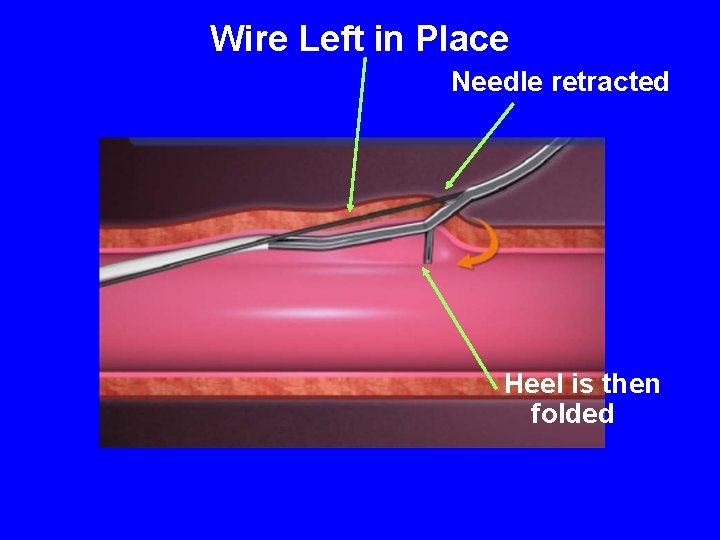
Wire Left in Place Needle retracted Heel is then folded

Device Removed

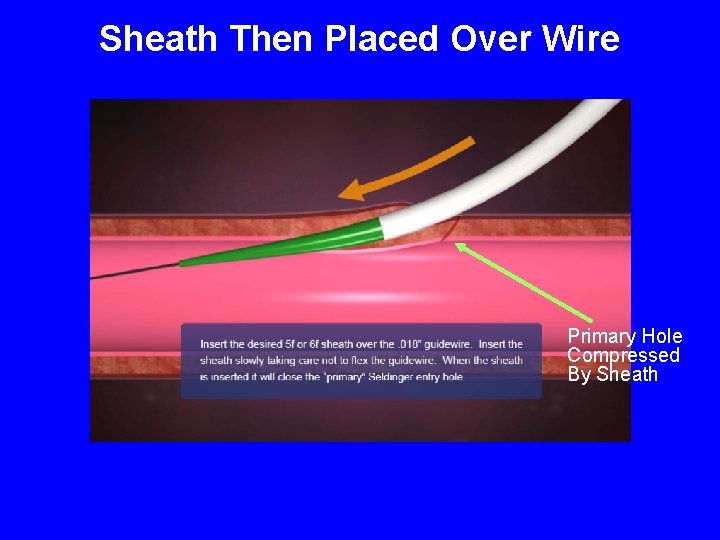
Sheath Then Placed Over Wire Primary Hole Compressed By Sheath

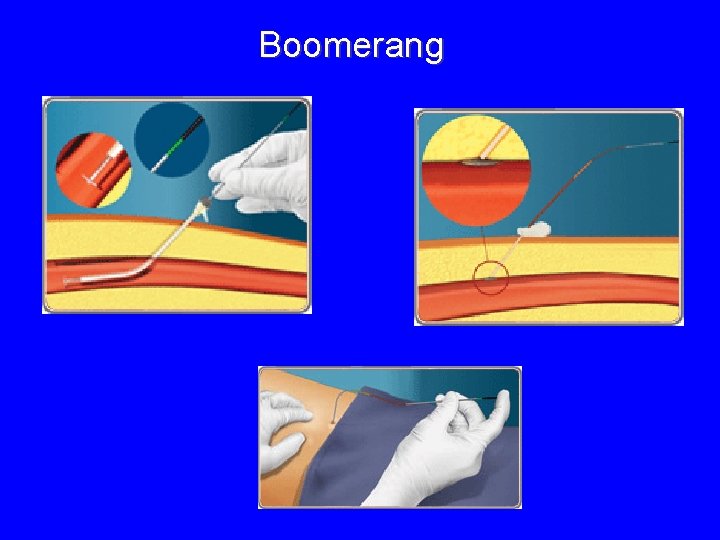
Boomerang

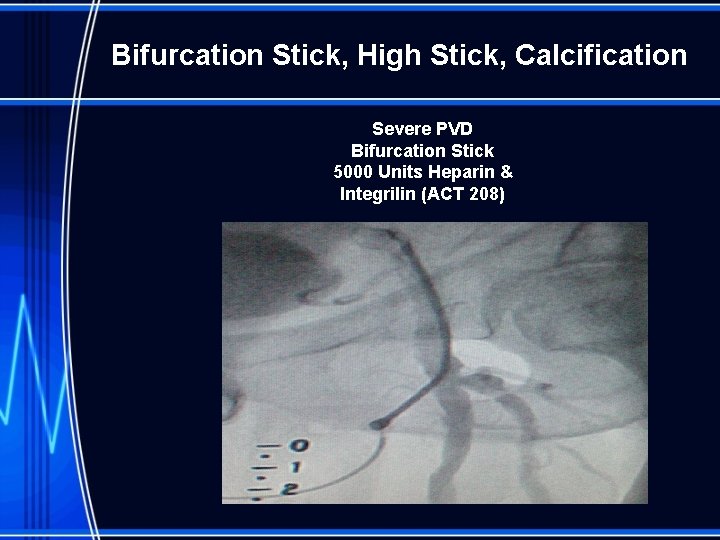
Bifurcation Stick, High Stick, Calcification Severe PVD Bifurcation Stick 5000 Units Heparin & Integrilin (ACT 208)

Conclusion • With new generations of VCD extravascular devices are beginning to demonstrate safety and efficacy similar or better to other devices leaving nothing behind. • Safety advantages are seen with extravascular devices. • Expanding closure to PAD patietns with calcification, Bifurcation, Large vessel holes, and high sticks are what is needed.