AC corrosion problems measures and findings Main topics

AC corrosion problems, measures and findings
Main topics of the presentation Why does alternating current (AC) corrosion exist Criteria for assessing the AC corrosion risk Measures to reduce the AC corrosion risk Calculation of the induction voltage through simulation Conclusions 12. 09. 2008, DI(FH) Johann Haberl 2
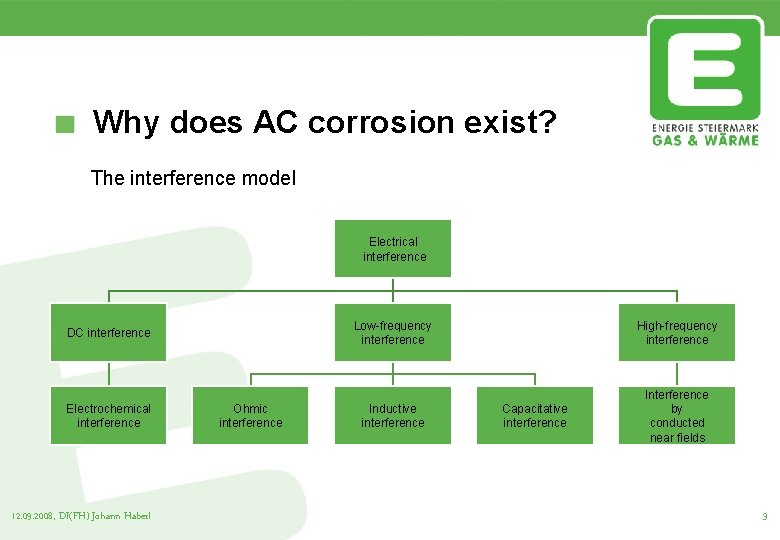
Why does AC corrosion exist? The interference model Electrical interference DC interference Electrochemical interference 12. 09. 2008, DI(FH) Johann Haberl Ohmic interference Low-frequency interference High-frequency interference Inductive interference Interference by conducted near fields Capacitative interference 3
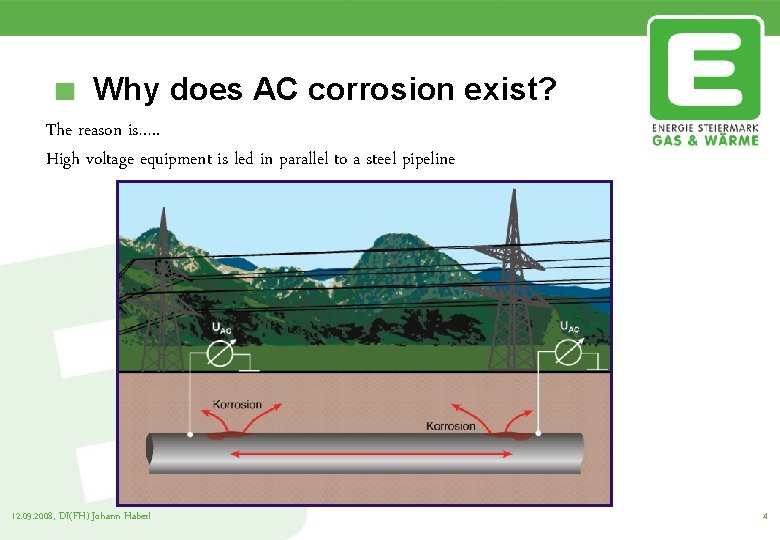
Why does AC corrosion exist? The reason is…. . High voltage equipment is led in parallel to a steel pipeline 12. 09. 2008, DI(FH) Johann Haberl 4
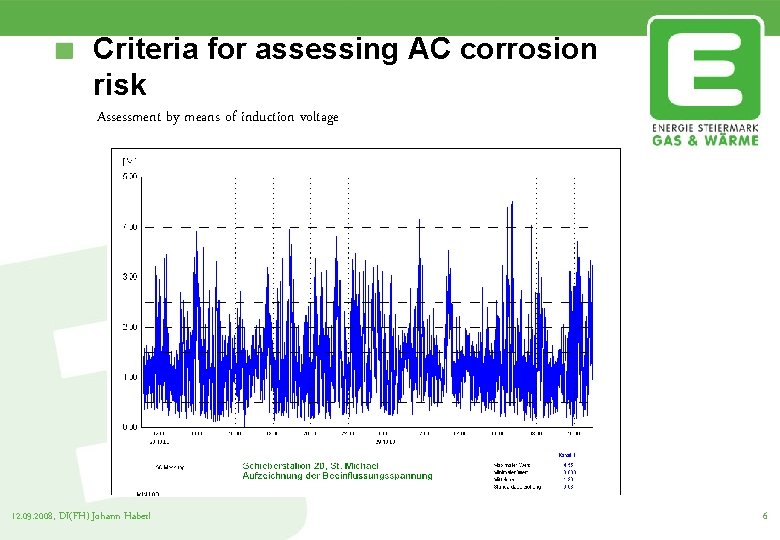
Criteria for assessing AC corrosion risk Assessment by means of induction voltage Measurement of the induced voltage through neutral earth Record the induced voltage with a sampling rate of 0, 5 seconds Voltages < 10 V no corrosion risk Voltages from 10 V up to 30 V corrosion risk exists Voltages > 30 V reduce the voltage 12. 09. 2008, DI(FH) Johann Haberl 5
Criteria for assessing AC corrosion risk Assessment by means of induction voltage 12. 09. 2008, DI(FH) Johann Haberl 6

Criteria for assessing AC corrosion risk Assessment by means of current density Install a steel test sheet with 1 cm² defect size Connection between test sheet and the pipeline Record the AC current from the test sheet Current densities > 30 A/m² corrosion risk exists 12. 09. 2008, DI(FH) Johann Haberl 7

Criteria for assessing AC corrosion risk Assessment by means of current density 12. 09. 2008, DI(FH) Johann Haberl 8
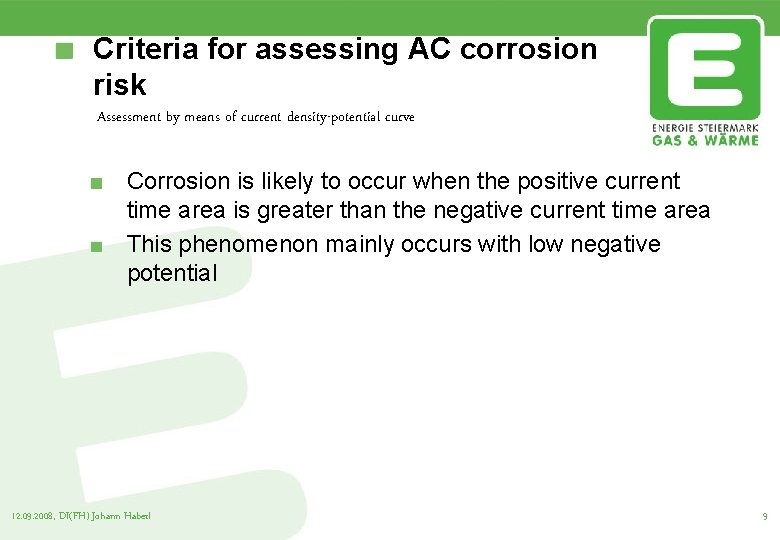
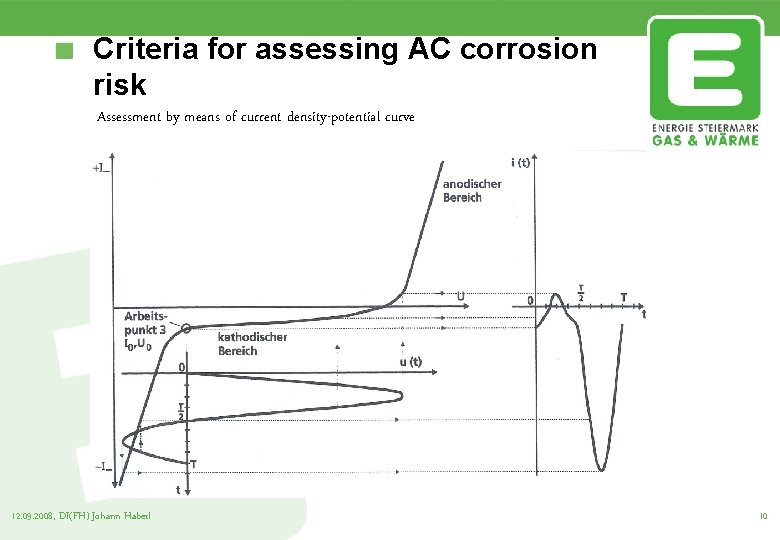
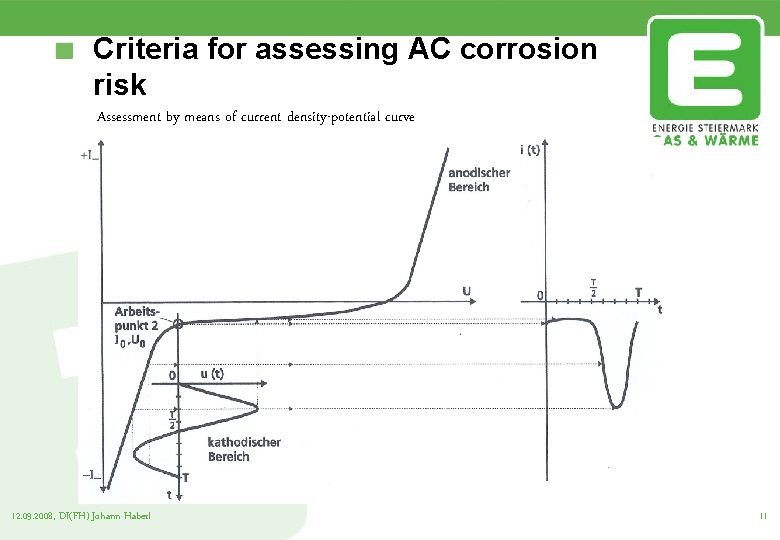
Criteria for assessing AC corrosion risk Assessment by means of current density-potential curve Corrosion is likely to occur when the positive current time area is greater than the negative current time area This phenomenon mainly occurs with low negative potential 12. 09. 2008, DI(FH) Johann Haberl 9
Criteria for assessing AC corrosion risk Assessment by means of current density-potential curve 12. 09. 2008, DI(FH) Johann Haberl 10
Criteria for assessing AC corrosion risk Assessment by means of current density-potential curve 12. 09. 2008, DI(FH) Johann Haberl 11
Measures to reduce the AC corrosion risk Polarisation cells Electrochemical component Electrodes are immersed in a 30% KOH solution A high DC resistance A low AC resistance High maintenance requirements 12. 09. 2008, DI(FH) Johann Haberl 12

Measures to reduce the AC corrosion risk Polarisation cells 12. 09. 2008, DI(FH) Johann Haberl 13
Measures to reduce the AC corrosion risk Power capacities As effective as polarisation cells Low maintenance requirements 12. 09. 2008, DI(FH) Johann Haberl 14

Measures to reduce the AC corrosion risk Power capacities 12. 09. 2008, DI(FH) Johann Haberl 15
Measures to reduce the AC corrosion risk Diode separating units The unit consists of four series connected power diodes in the negative leg and one back to back power diode in the positive leg The unit delimits AC voltages in positive half wave to +0, 7 V and to -2, 8 V in negative half wave 12. 09. 2008, DI(FH) Johann Haberl 16
Measures to reduce the AC corrosion risk Partial compensation Compensation of the positive half-wave of the inducted voltage There are two compensation systems necessary The DC potential increases 12. 09. 2008, DI(FH) Johann Haberl 17
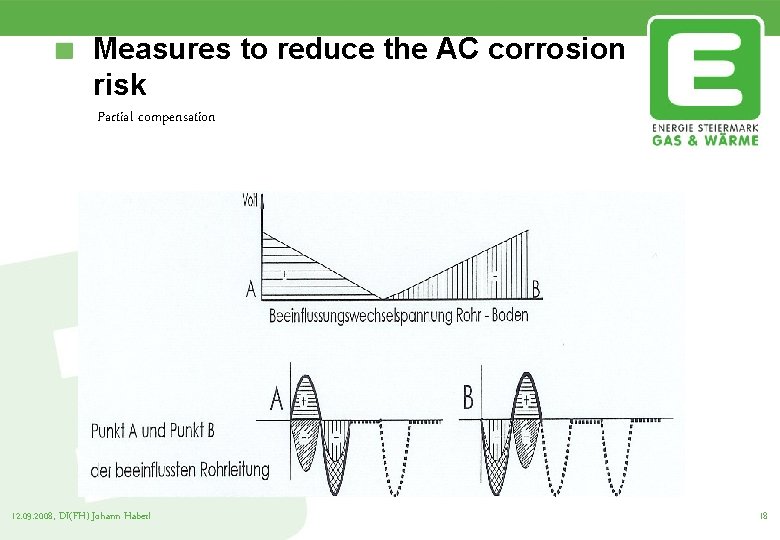
Measures to reduce the AC corrosion risk Partial compensation 12. 09. 2008, DI(FH) Johann Haberl 18

Measures to reduce the AC corrosion risk Reduction of the cathodic protection sections It would be very effective To split one large cathodic protection system into many short systems Reduce the length of the steel pipeline which is led in parallel to the high voltage equipment 12. 09. 2008, DI(FH) Johann Haberl 19
The simulation model The impedance model A mathematical simulation model consists of A steel pipeline and components to reduce the induction voltage It shows the results of damages in the insulation 12. 09. 2008, DI(FH) Johann Haberl 20
The simulation model The idea behind the simulation To link – Data of the piping system – Data of high voltage equipment – Data of chemical parts involved And to receive as an output A prediction of possible weak points, which could cause AC- corrosion as a consequence 12. 09. 2008, DI(FH) Johann Haberl 21
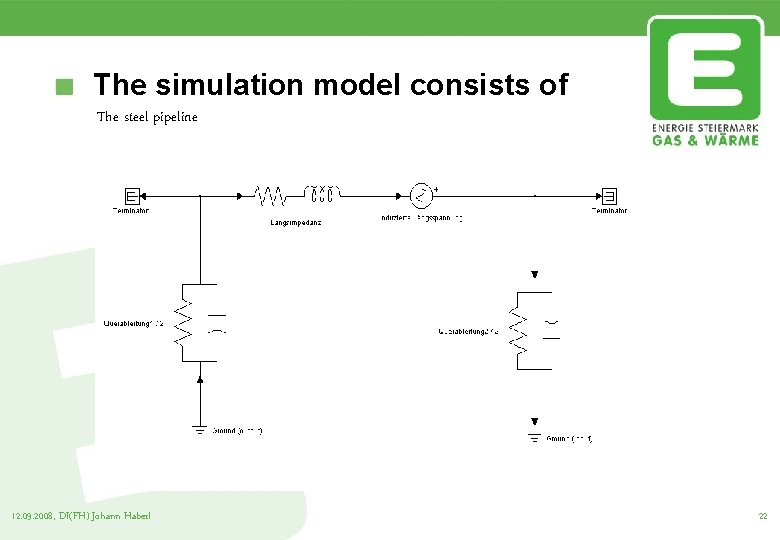
The simulation model consists of The steel pipeline 12. 09. 2008, DI(FH) Johann Haberl 22
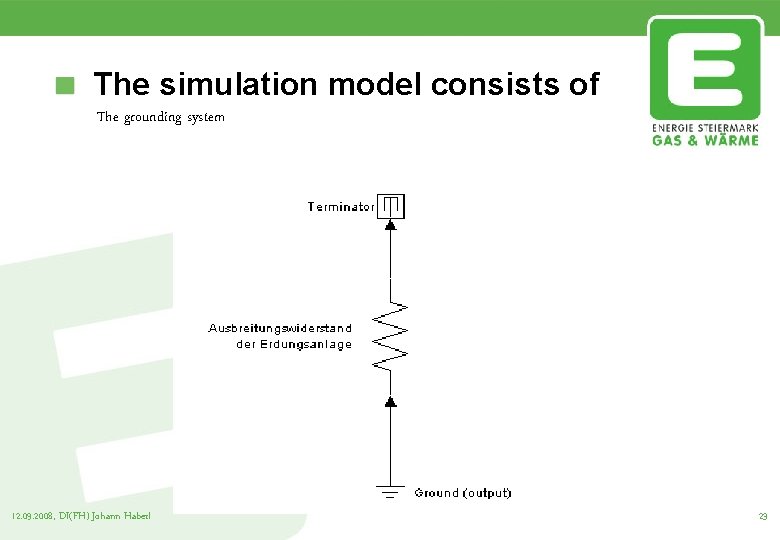
The simulation model consists of The grounding system 12. 09. 2008, DI(FH) Johann Haberl 23
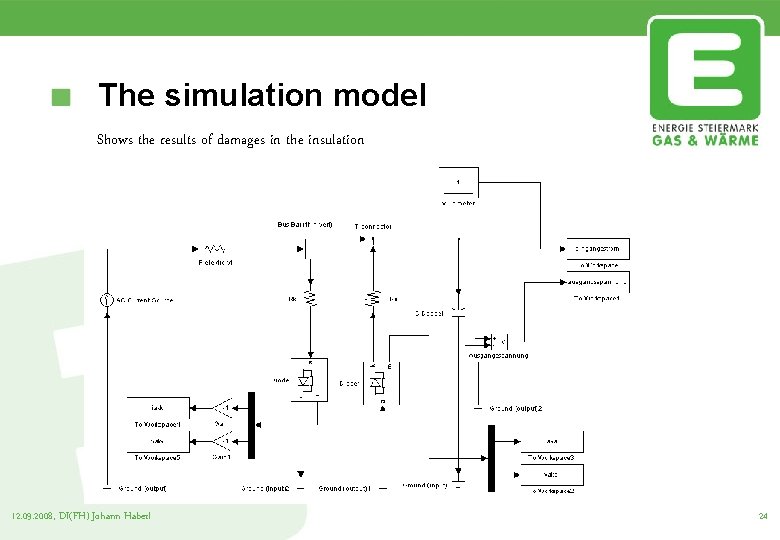
The simulation model Shows the results of damages in the insulation 12. 09. 2008, DI(FH) Johann Haberl 24

The simulation model Increase the accuracy by dividing the steel pipeline into dedicated areas 12. 09. 2008, DI(FH) Johann Haberl 25
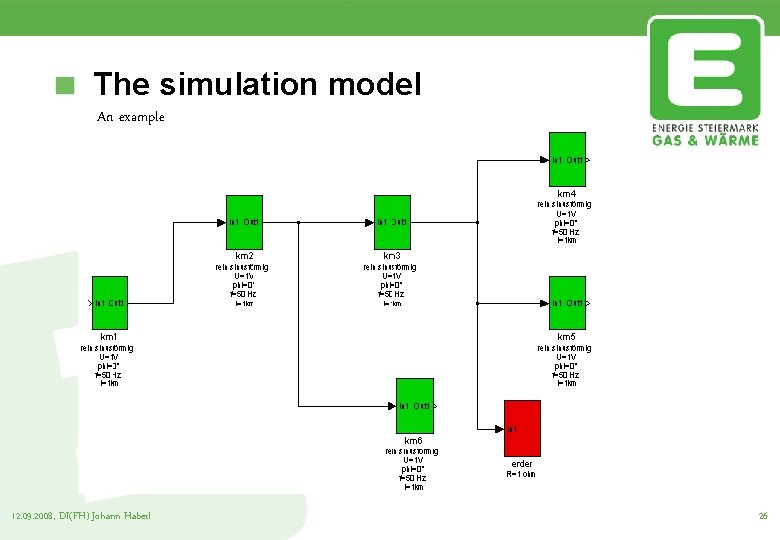
The simulation model An example 12. 09. 2008, DI(FH) Johann Haberl 26
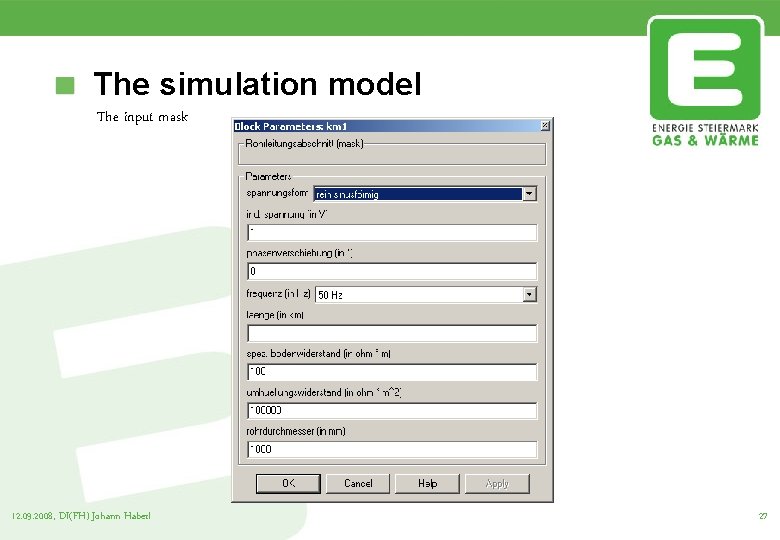
The simulation model The input mask 12. 09. 2008, DI(FH) Johann Haberl 27
Results and prospects A simulation model with high accuracy A prediction of weak points in a steel pipeline Next steps are To make the model more comfortable To get deeper knowledge in chemical reaction causing AC corrosion 12. 09. 2008, DI(FH) Johann Haberl 28

Thanks for your attention Much Engerie
- Slides: 29