Absorption Stripping Typical Absorber Solvent water 1943 kgmol

Absorption / Stripping
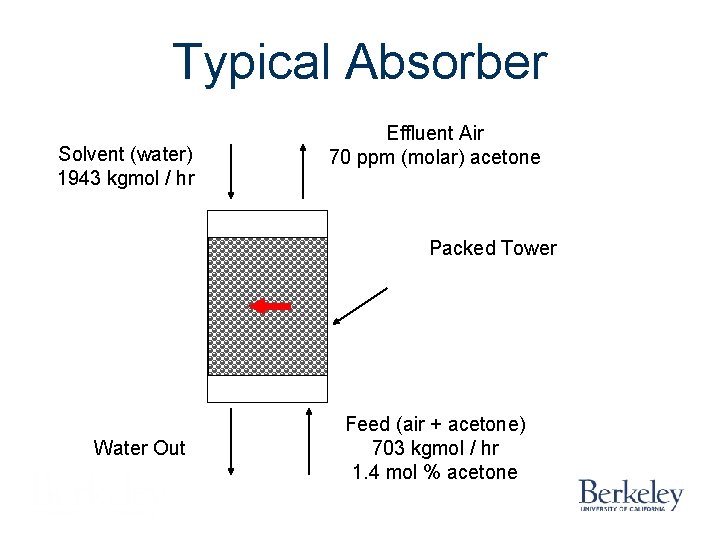
Typical Absorber Solvent (water) 1943 kgmol / hr Effluent Air 70 ppm (molar) acetone Packed Tower Water Out Feed (air + acetone) 703 kgmol / hr 1. 4 mol % acetone
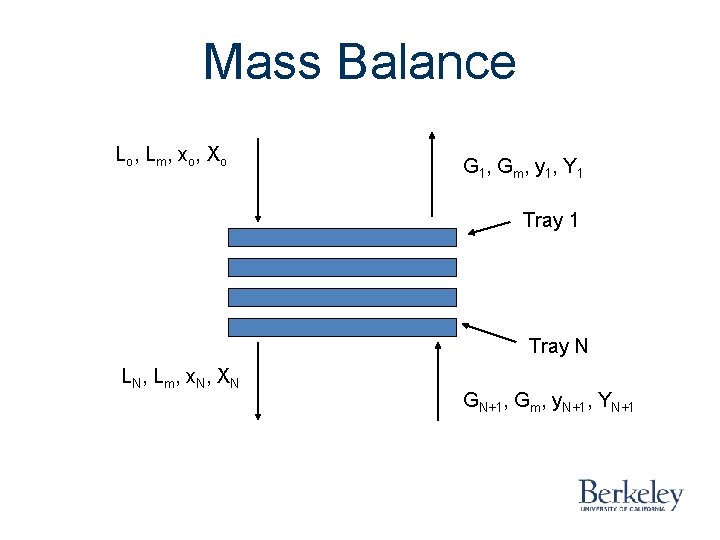
Mass Balance Lo , L m , x o , X o G 1 , G m, y 1 , Y 1 Tray N LN, Lm, x. N, XN GN+1, Gm, y. N+1, YN+1
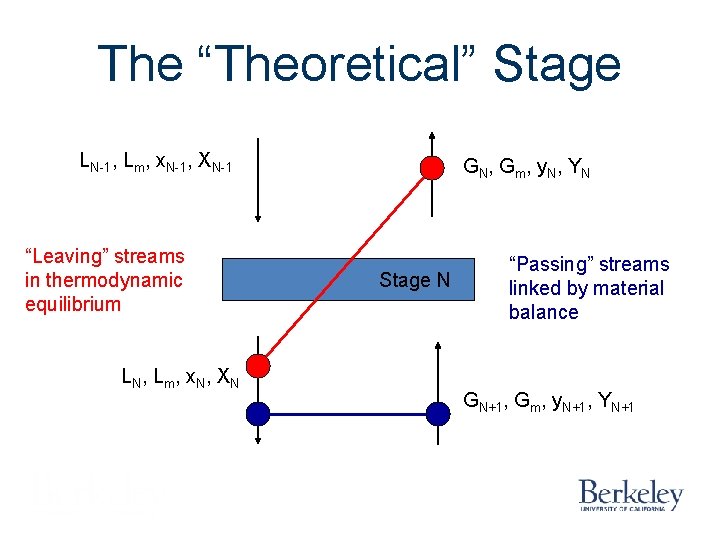
The “Theoretical” Stage LN-1, Lm, x. N-1, XN-1 “Leaving” streams in thermodynamic equilibrium LN, Lm, x. N, XN GN, Gm, y. N, YN Stage N “Passing” streams linked by material balance GN+1, Gm, y. N+1, YN+1

Absorber Operating Line Solvent 200 80/4 4 CO 2 200/4 Molar Flows 80/8 4 CO 2 200/8 Tray 3 80/16 4 CO 2 Solvent / CO 2 200/16 Tray 2 80/12 4 CO 2 200/12 Tray 1 80/20 Tray 4 N 2 / CO 2
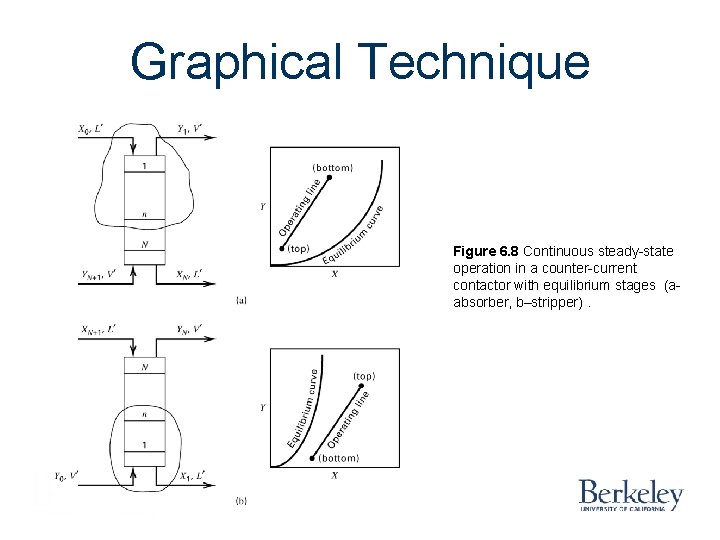
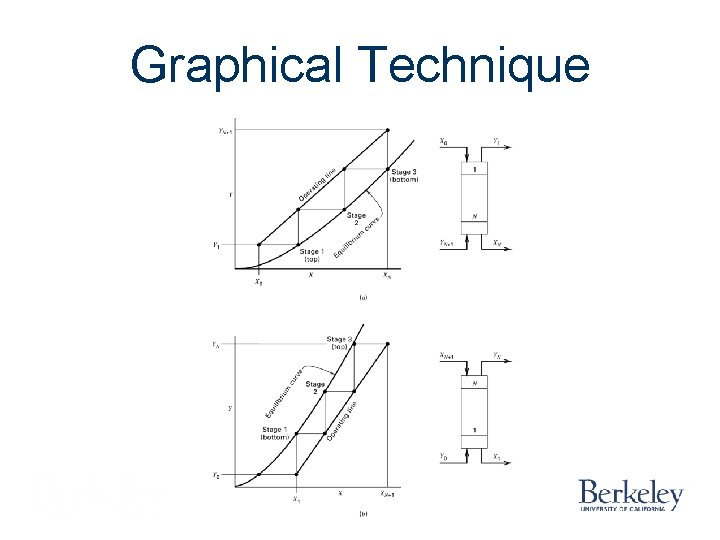
Graphical Technique Figure 6. 8 Continuous steady-state operation in a counter-current contactor with equilibrium stages (aabsorber, b–stripper).

Graphical Technique Figure 6. 9 Operating lines for an absorber
Graphical Technique

Kremser Method for Theoretical Trays (Absorber) Lo , x o G 1, y 1 Tray N LN, x. N GN+1, y. N+1
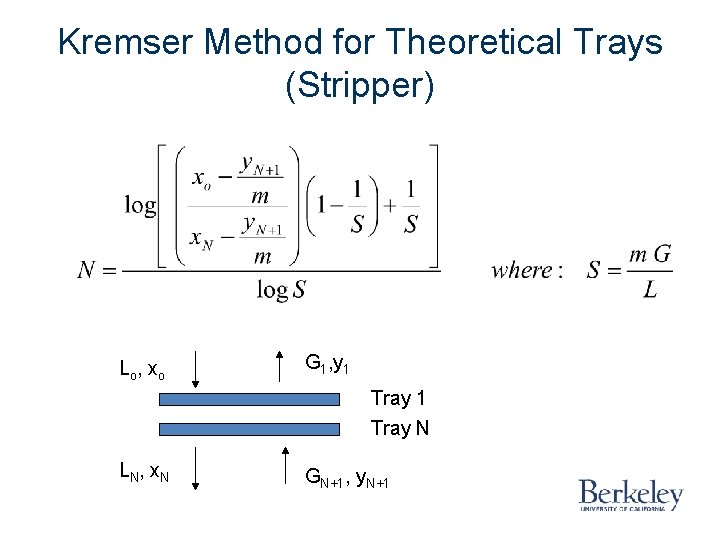
Kremser Method for Theoretical Trays (Stripper) Lo , x o G 1, y 1 Tray N LN, x. N GN+1, y. N+1

Kremser Chart

Example Carbon Disulfide, CS 2, used as a solvent in a chemical plant, is evaporated from the product in a drier into an inert gas (essentially N 2) in order to avoid an explosion hazard. The vapor-N 2 mixture is to be scrubbed with an absorbent oil, which will be subsequently steam stripped to recover the CS 2. The CS 2 -N 2 mixture has a partial pressure of CS 2 equal to 50 mm Hg at 24 C and is to be blown into the absorber at essentially standard atmospheric pressure at the rate of 50, 000 ft 3 / hr. The vapor content of the gas is to be reduced to 0. 5 %. The absorption oil has a molecular weight of 180. The oil enters the absorber free of CS 2. The oil – CS 2 solution follows Raoult’s Law. The vapor pressure of CS 2 at 24 C is 346 mm Hg. Determine: The minimum liquid / gas ratio For a liquid / gas ratio of 1. 5 times the minimum, determine the flow rate of oil in lb / hr The required theoretical stages graphically and from the Kremser method.

Packed Tower Design Random Packing

Structured Packing Fair, J. R. , Seibert, A. F. , Behrens, M. , Saraber, P. P. , and Olujic, Z. “Structured Packing Performance-Experimental Evaluation of Two Predictive Models ”, Ind. Eng. Chem. Res. 39 (6), 1788 -1796 (2000).

Structured Packing
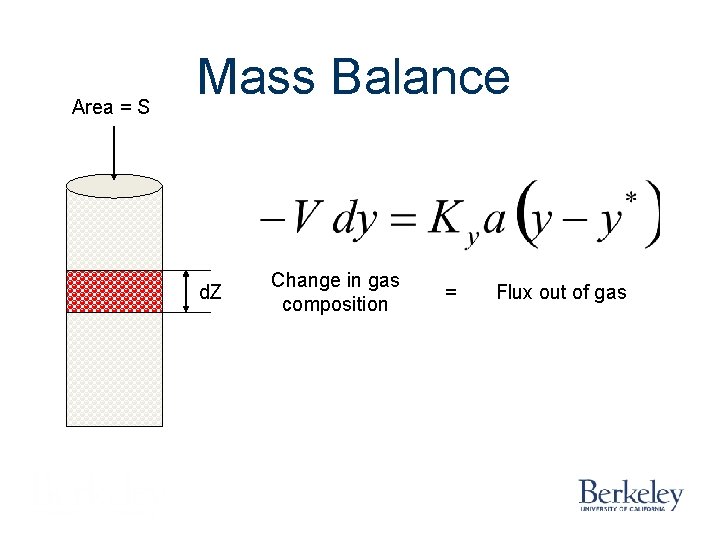
Area = S Mass Balance d. Z Change in gas composition = Flux out of gas
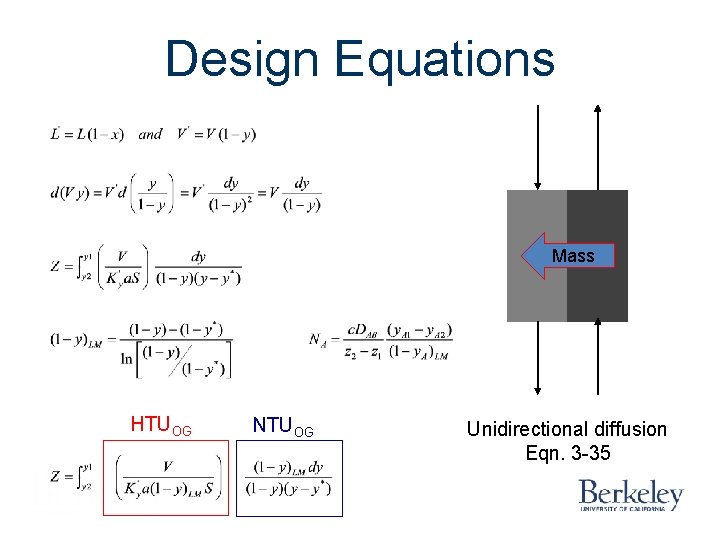
Design Equations Mass HTUOG NTUOG Unidirectional diffusion Eqn. 3 -35
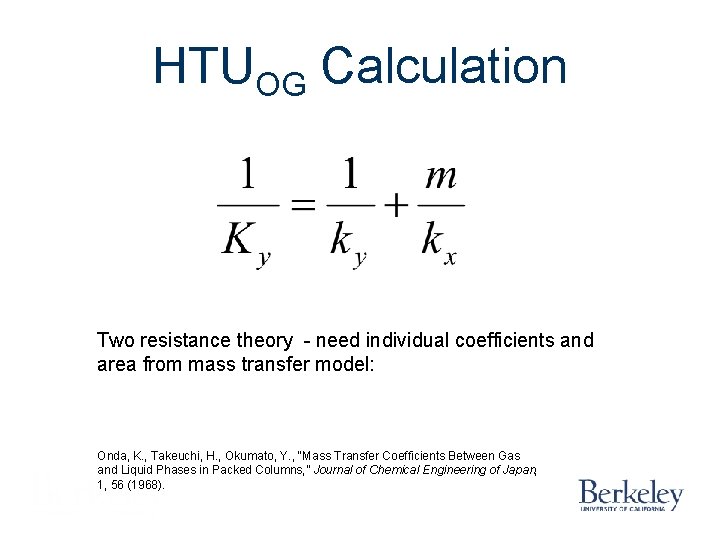
HTUOG Calculation Two resistance theory - need individual coefficients and area from mass transfer model: Onda, K. , Takeuchi, H. , Okumato, Y. , “Mass Transfer Coefficients Between Gas and Liquid Phases in Packed Columns, ” Journal of Chemical Engineering of Japan, 1, 56 (1968).
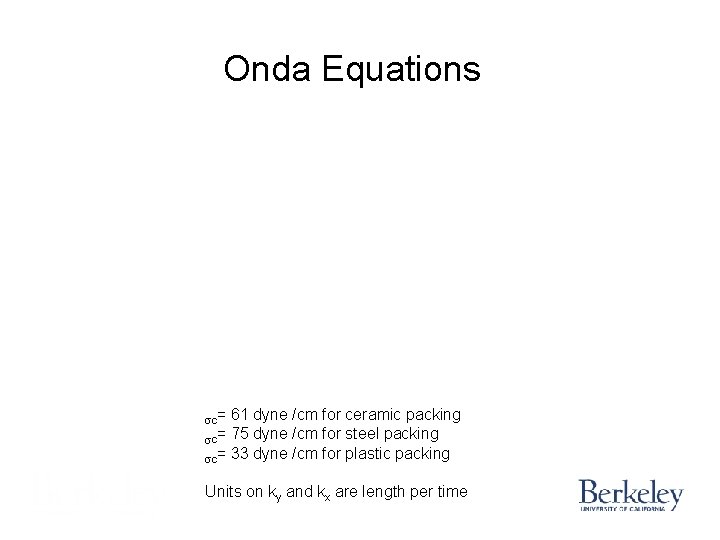
Onda Equations sc= 61 dyne /cm for ceramic packing sc= 75 dyne /cm for steel packing sc= 33 dyne /cm for plastic packing Units on ky and kx are length per time
Effective Area (aw)

Structured Packing Wetted Area
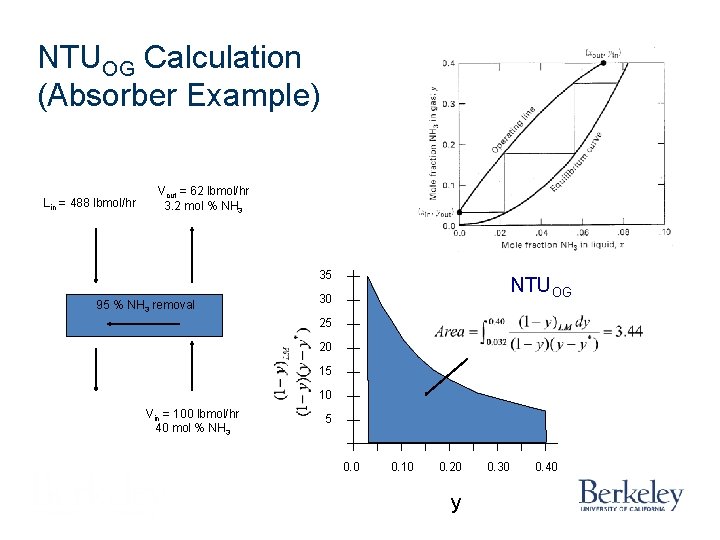
NTUOG Calculation (Absorber Example) Lin = 488 lbmol/hr Vout = 62 lbmol/hr 3. 2 mol % NH 3 35 95 % NH 3 removal NTUOG 30 25 20 15 10 Vin = 100 lbmol/hr 40 mol % NH 3 5 0. 0 0. 10 0. 20 y 0. 30 0. 40
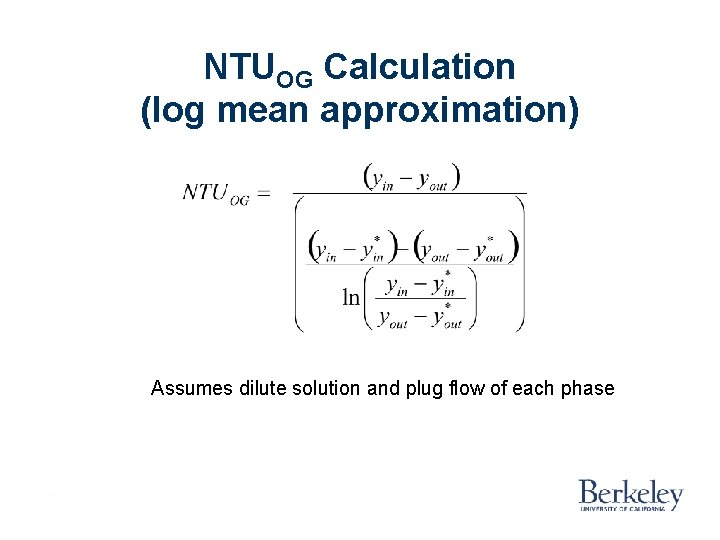
NTUOG Calculation (log mean approximation) Assumes dilute solution and plug flow of each phase

Packed Column HETP Behavior
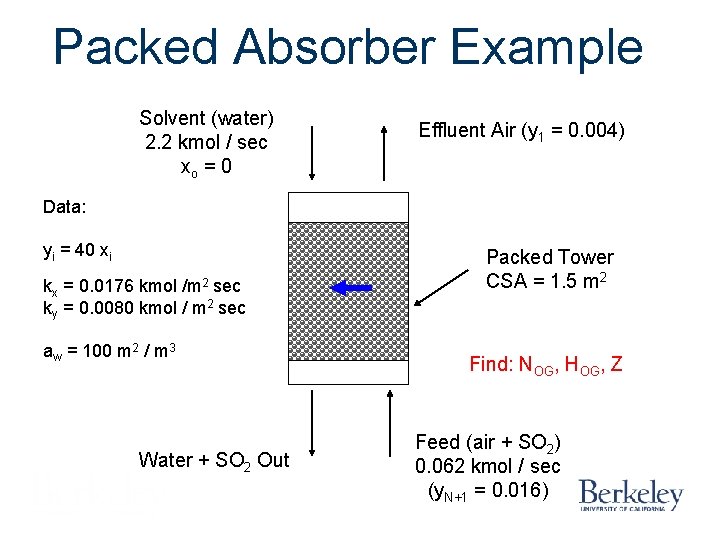
Packed Absorber Example Solvent (water) 2. 2 kmol / sec xo = 0 Effluent Air (y 1 = 0. 004) Data: yi = 40 xi kx = 0. 0176 kmol /m 2 sec ky = 0. 0080 kmol / m 2 sec aw = 100 m 2 / m 3 Water + SO 2 Out Packed Tower CSA = 1. 5 m 2 Find: NOG, HOG, Z Feed (air + SO 2) 0. 062 kmol / sec (y. N+1 = 0. 016)

10 Minute Problem A packed absorption tower yields a concentration based. NTUOG of 10. Determine the tower height given the following information (The concentrations of the transferred component are dilute in both the liquid and gas). Data: Tower diameter = 5 ft. Gas density = 0. 20 lb/ ft 3 Gas superficial velocity = 3 ft / sec Kog = 0. 01 m /sec m = 2. 0 P = 1 atm, T = 80 F ae = 200 m 2 /m 3

Packed Tower Hydraulics (random packing) 1) Leva plot technique 2) Stichlmair model
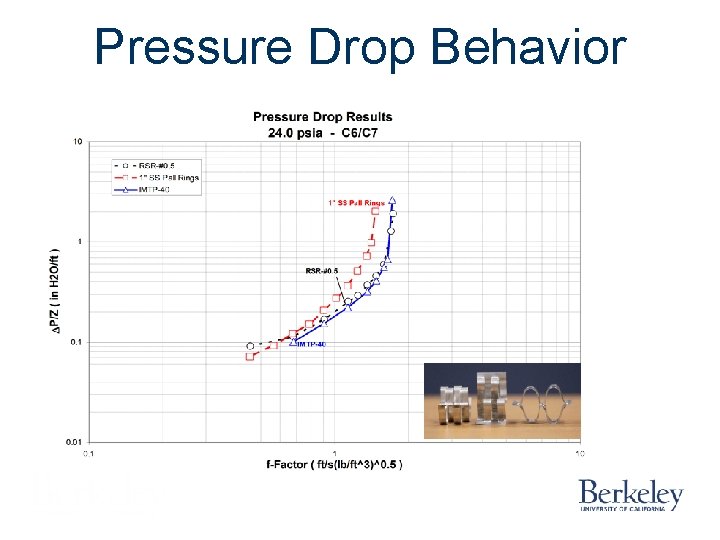
Pressure Drop Behavior
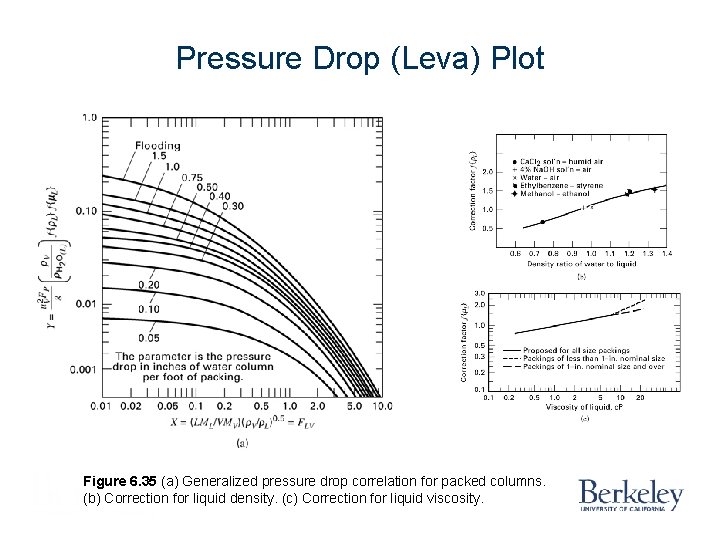
Pressure Drop (Leva) Plot Figure 6. 35 (a) Generalized pressure drop correlation for packed columns. (b) Correction for liquid density. (c) Correction for liquid viscosity.

Packing Factors
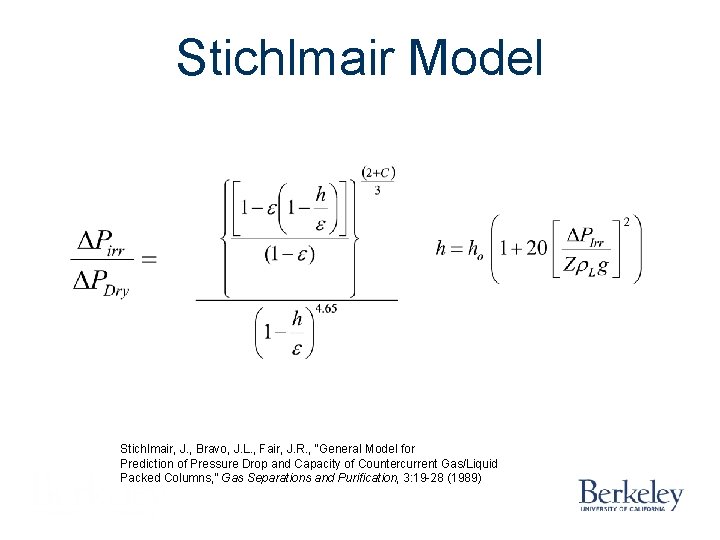
Stichlmair Model Stichlmair, J. , Bravo, J. L. , Fair, J. R. , “General Model for Prediction of Pressure Drop and Capacity of Countercurrent Gas/Liquid Packed Columns, ” Gas Separations and Purification, 3: 19 -28 (1989)
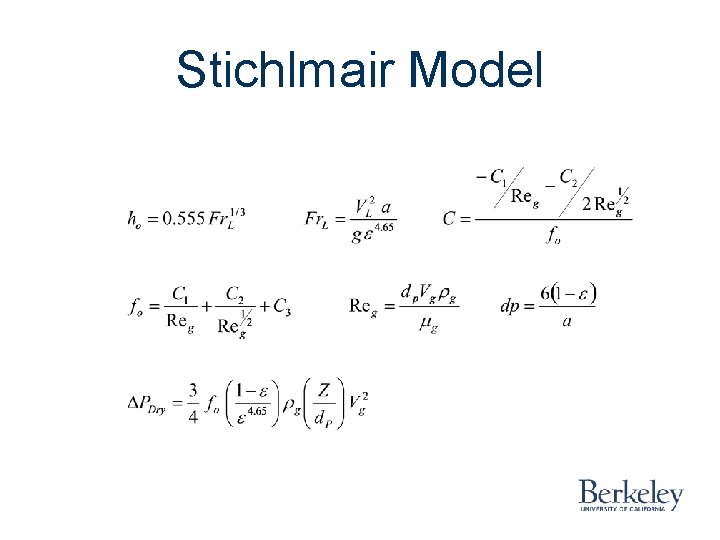
Stichlmair Model
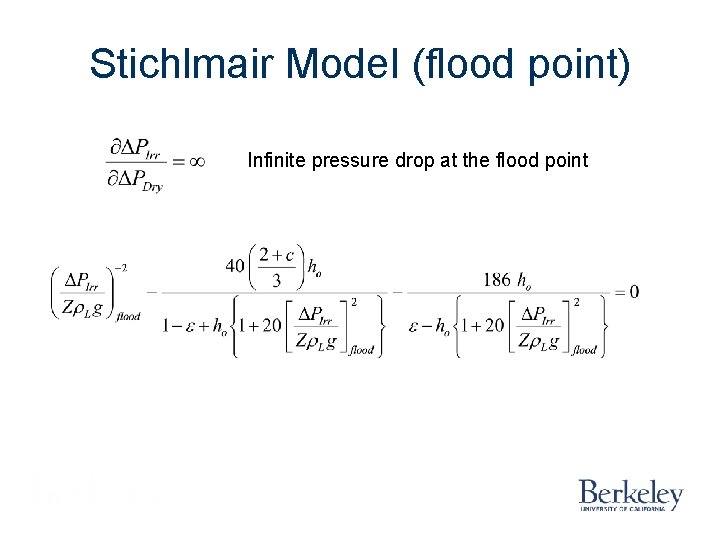
Stichlmair Model (flood point) Infinite pressure drop at the flood point
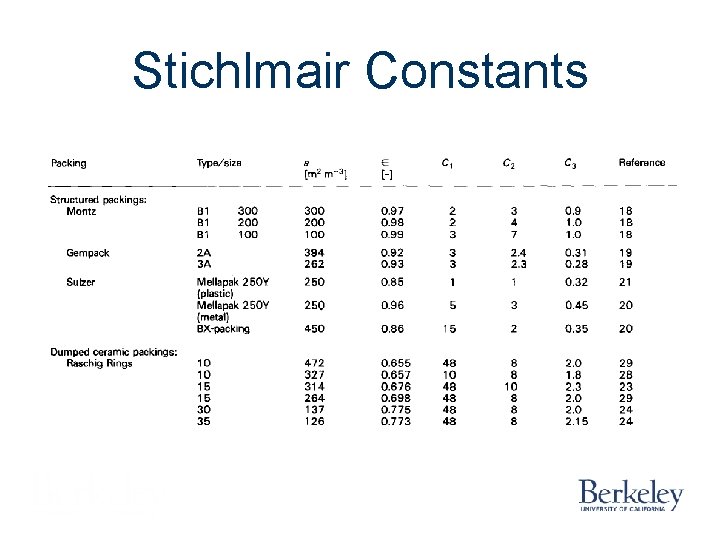
Stichlmair Constants
Example A tower packed with 1 in. Ceramic Intalox saddles is to be built to treat 25, 000 ft 3/ hr of entering gas. The ammonia content of the entering gas is 2 percent by volume. Ammonia-free water is used as the absorbent. The temperature is 68 F and the pressure is 1 atm. The ratio of the gas flow to the liquid flow is 1 lb of gas per lb of liquid. The tower diameter is 1. 67 ft. Using both the Leva plot and the Stichlmair correlation determine the total tower pressure drop if the tower packed height is 20 feet.

10 Minute Problem An air-water test is being run on a 10 foot bed of 1 inch (25 mm) metal Pall rings. The air rate is 350 ft 3 / min and the liquid rate is 15 GPM / ft 2. The tower is 16. 8 inches in diameter, the operating pressure is 1 atm and the temperature is 80 F. What is the total column pressure drop?
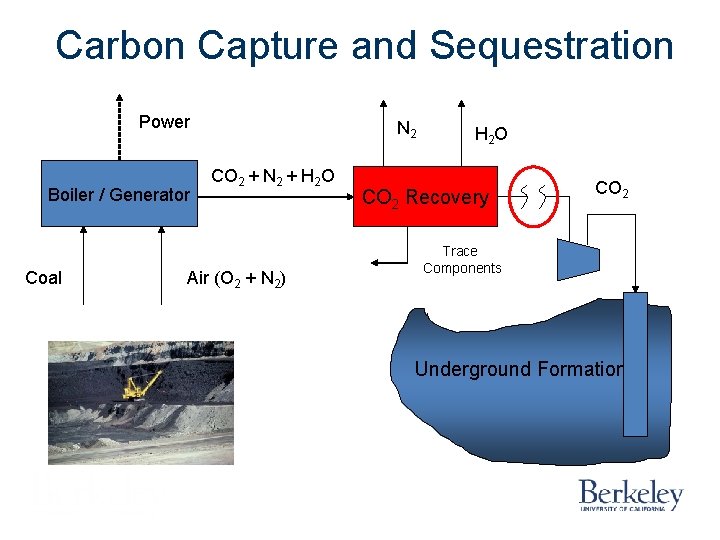
Carbon Capture and Sequestration Power Boiler / Generator Coal N 2 CO 2 + N 2 + H 2 O Air (O 2 + N 2) H 2 O CO 2 Recovery CO 2 Trace Components Underground Formation

Absorption with Chemical Reaction Flue Gas Out 40– 65 °C CO 2 Absorber Heat X Stripper Flue Gas In 5 % O 2 12 % CO 2 83 % N 2 100– 120 °C Reboiler Rich Amine Lean Amine
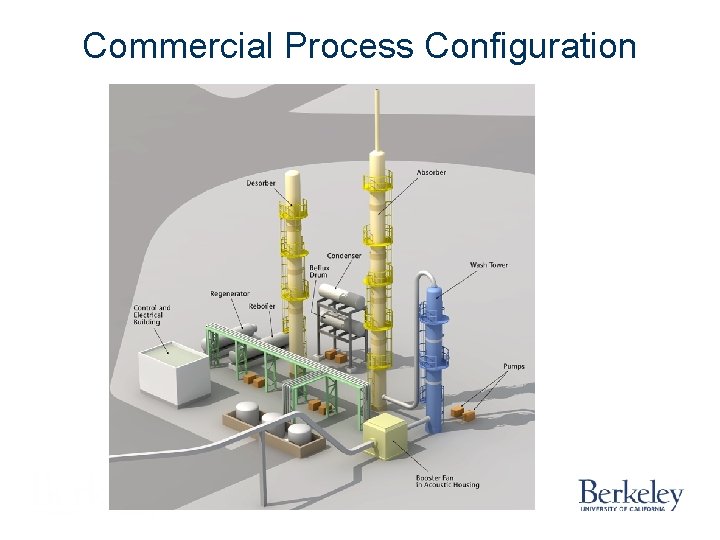
Commercial Process Configuration
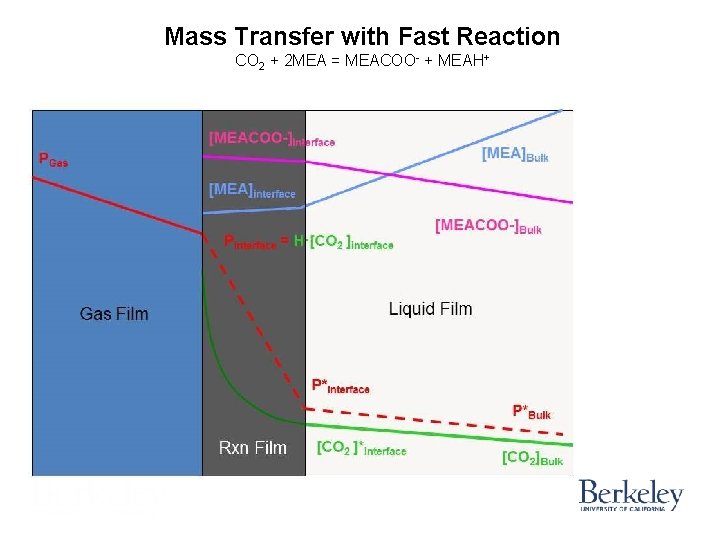
Mass Transfer with Fast Reaction CO 2 + 2 MEA = MEACOO- + MEAH+
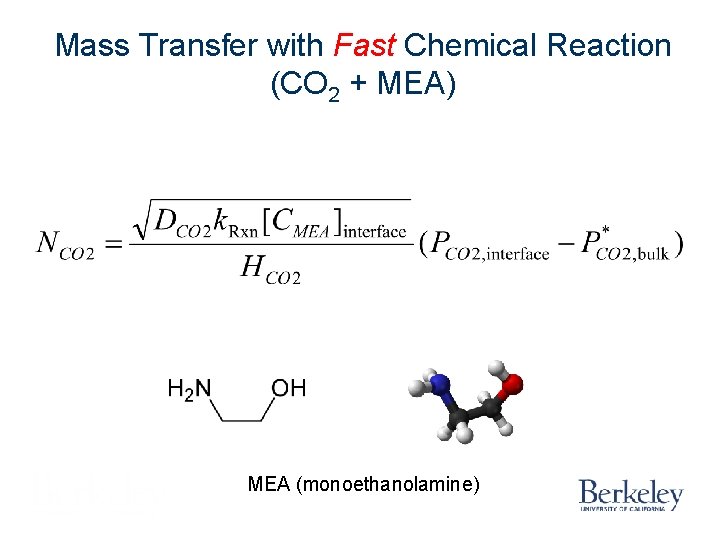
Mass Transfer with Fast Chemical Reaction (CO 2 + MEA) MEA (monoethanolamine)
- Slides: 41