Absolute Age Actual Age Radioactive Decay Nuclei of
Absolute Age Actual Age
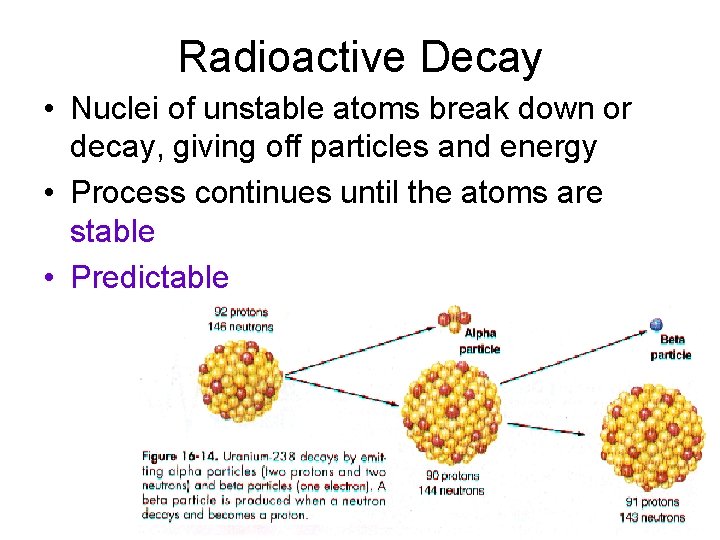
Radioactive Decay • Nuclei of unstable atoms break down or decay, giving off particles and energy • Process continues until the atoms are stable • Predictable

Parent Unstable Radioactive Isotope Daughter Stable Decay Product Carbon 14 (C 14) Nitrogen 14 (N 14) Uranium 238 (U 238) Lead 206 (Pb 206)
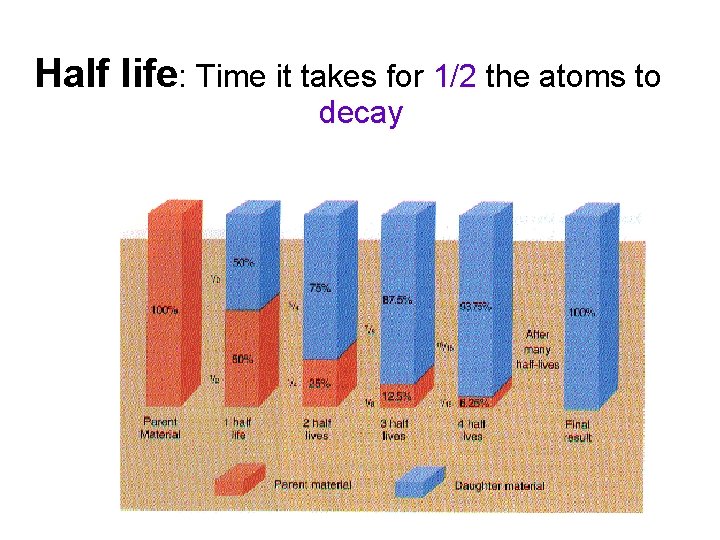
Half life: Time it takes for 1/2 the atoms to decay
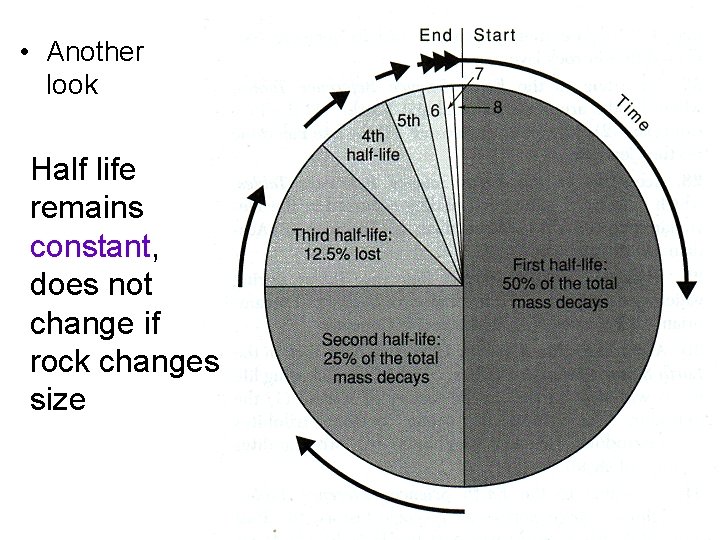
• Another look Half life remains constant, does not change if rock changes size
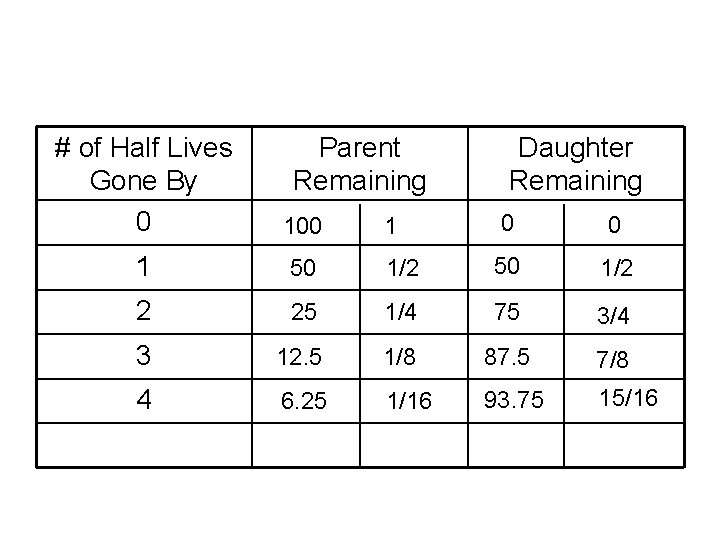
# of Half Lives Gone By 0 Parent Remaining 100 1 Daughter Remaining 1 0 0 50 1/2 2 25 1/4 75 3/4 3 12. 5 1/8 87. 5 7/8 4 6. 25 1/16 93. 75 15/16
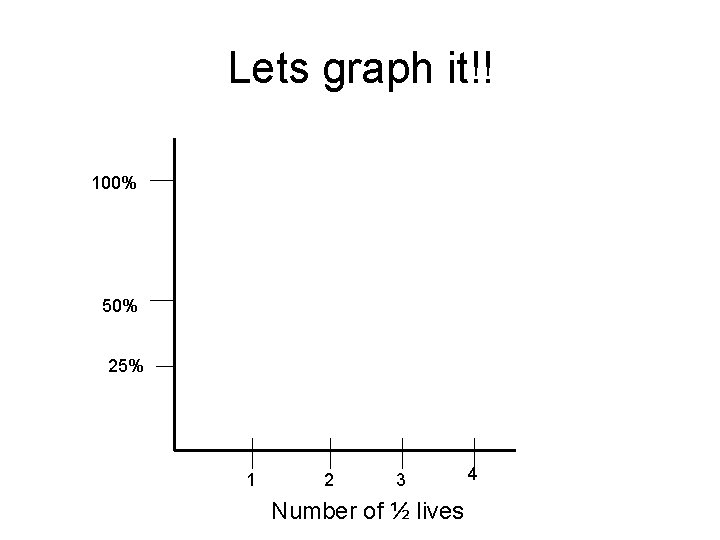
Lets graph it!! 100% 50% 25% 1 2 3 Number of ½ lives 4

Radiometric Dating Knowing the amounts of parent and daughter atoms and the half life of the radioactive atoms, you can determine the age of the object
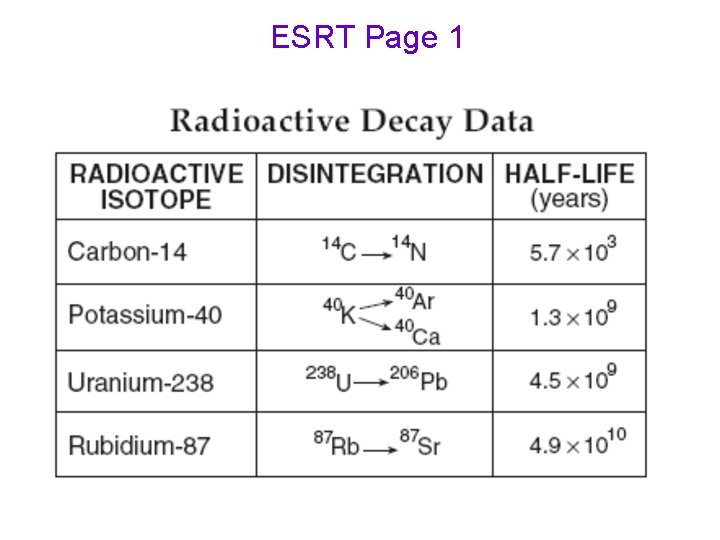
ESRT Page 1
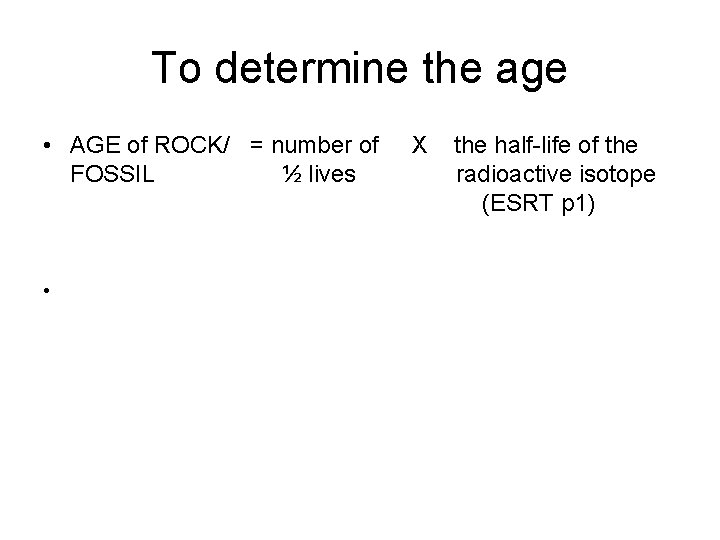
To determine the age • AGE of ROCK/ = number of X the half-life of the FOSSIL ½ lives radioactive isotope (ESRT p 1) •

Carbon 14 - Shorter ½ life - Used to date plants and animals of past 40, 000 years - Carbon is incorporated into the cells of living organisms and begins to decay when the organism dies

Uranium 238 Used to date older rocks Has a larger half life
- Slides: 12