Abnormal Uterine Bleeding structural cause Zahra Tavoli Assistant


Abnormal Uterine Bleeding structural cause Zahra Tavoli Assistant Professor Tehran University of Medical Science Department of Obstetrics and Gynecology Fellowship of laparoscopy
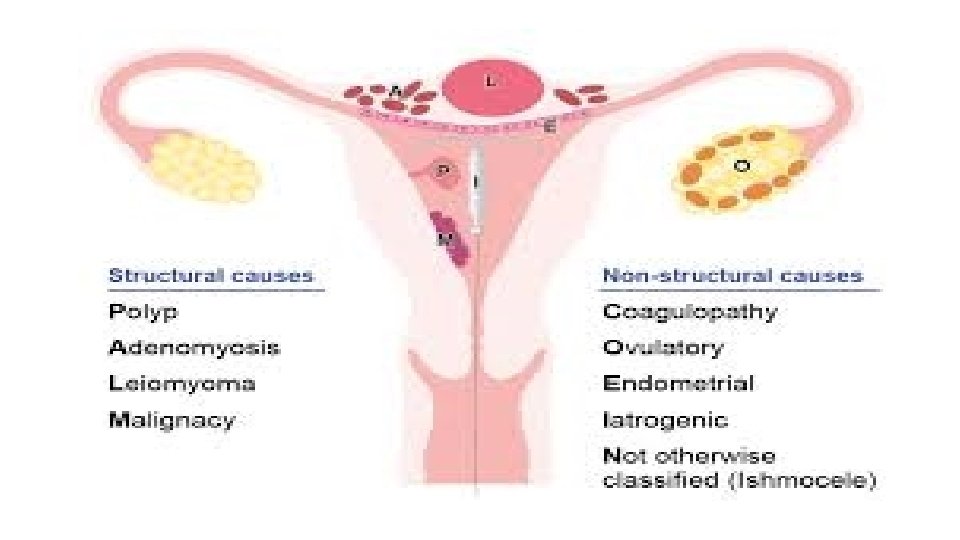
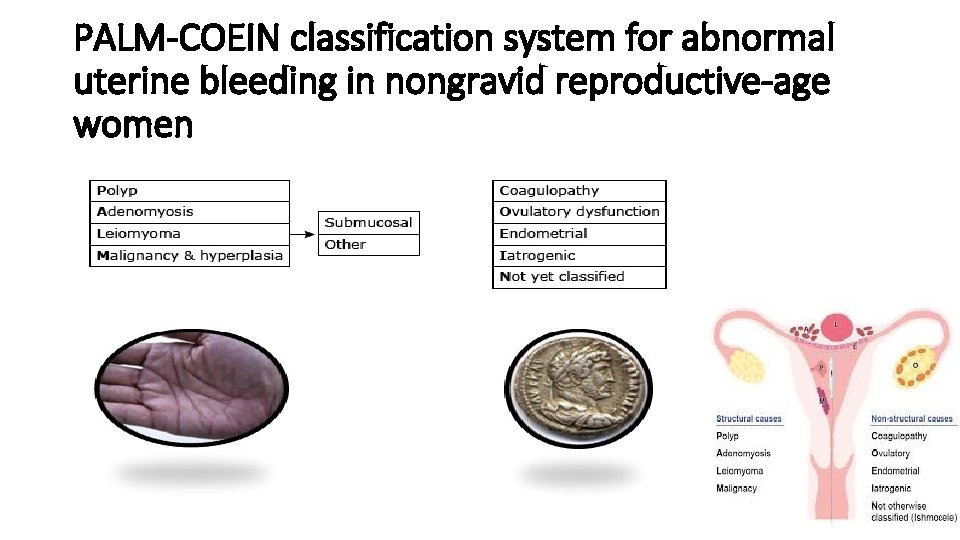
PALM-COEIN classification system for abnormal uterine bleeding in nongravid reproductive-age women
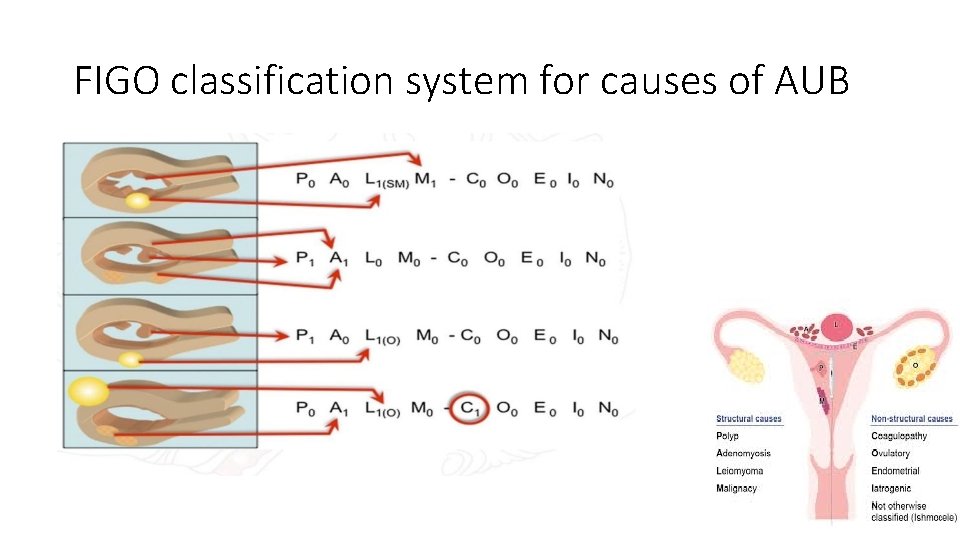
FIGO classification system for causes of AUB
PALM • P: Endometrial polyp IMB or PCB in 30 -50 year old woman • A: Adenomyosis – Dysmenorrhea, dyspareunia, chronic pelvic pain, sometimes menorrhagia • L: Leiomyoma – Submucous myoma – Menorrhagia; rarely IMB; never metrorrhagia AUB: Structural Conditions
PALM v M: Malignancy and hyperplasia ü Adenomatous hyperplasia (AH) -atypical AH-endometrial carcinoma • Post-menopausal bleeding • Recurrent perimenopausal metrorrhagia • Chronic anovulator (PCOS) with metrorrhagia – ü Leiomyosarcoma • Post-menopausal bleeding
Endometrial polyp • Hyperplastic overgrowths of endometrial glands and stroma • Around a vascular core that form a sessile or pedunculated projection • May also be asymptomatic • Often are benign, but malignancy occurs in some women. • Single or multiple • Size are from a few millimeters to several centime
Pathogenesis • Several molecular mechanisms üMonoclonal endometrial hyperplasia üOverexpression of endometrial aromatase üGene mutations • cytogenetic rearrangements • express both estrogen and progesterone receptors • progesterone may serve an antiproliferative function
Epidemiology • Rare among adolescents • The frequency is difficult to establish (because of asymptomatic) • increasing age • higher in postmenopausal • The prevalence during biopsy or hysterectomy is 10 to 24 percent
Clinical presentation abnormal uterine bleeding- 64 to 88 percent Postmenopausal bleeding and breakthrough bleeding during HRT asymptomatic discovered as the result of an evaluation for infertility a finding of endometrial cells on cervical cytology an incidental finding on endometrial sampling pelvic imaging, or hysteroscopy prolapse of the polyp at the external cervical
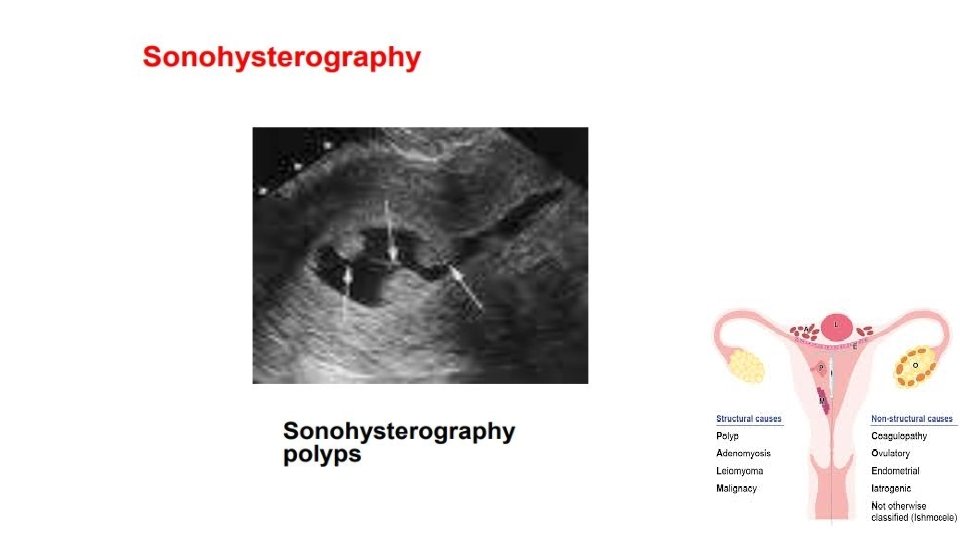
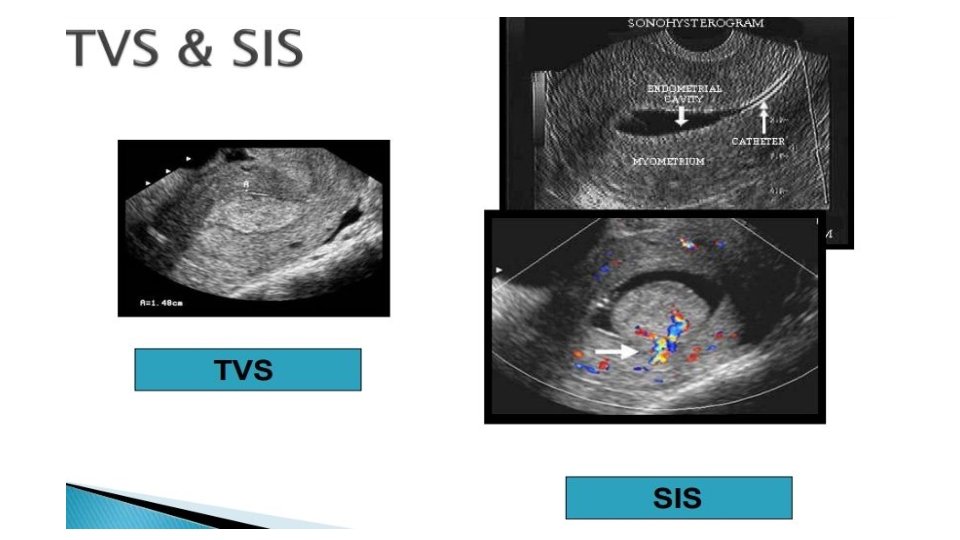
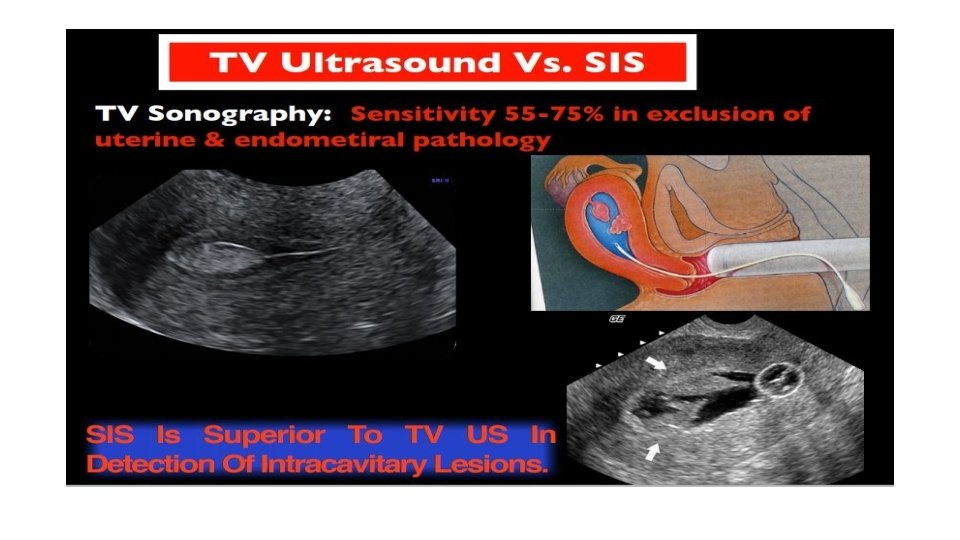
Diagnostic evaluation • Physical examination üNo physical examination findings except in a prolapsed polyp üSoft and friable consistency • Pelvic ultrasound and hysteroscopy • Transvaginal ultrasound (TVUS) is the first line and choice • Histologic diagnosis that can also exclude malignancy • Office endometrial biopsy (polyp may not have been) removed • Polypectomy performed for symptom relief or to exclude malignancy
Uterine adenomyosis • Endometrial glands and stroma are present within the myometrium • Hypertrophy of the surrounding myometrium. • Women with symptomatic adenomyosis present with • Uterine enlargement • Abnormal uterine bleeding • Painful menses.
Histopathology • Diffuse adenomyosis • Uniformly enlarged and boggy • In contrast to the irregular and firm appearance of the fibroid uterus • concurrently with fibroid • The average uterine weight 80 to 200 grams • Myometrial wall appears thickened • Contains small hemorrhagic or chocolate-colored areas • representing islands of endometrial bleeding.
Histopathology • Focal adenomyosis (also called adenomyoma) üResemble a fibroid but without the pseudocapsule üNot allows for easy enucleation • Juvenile cystic adenomyoma ü 30 years or younger üSevere dysmenorrhea üMyometrial cysts ≥ 1 cm. • Cystic adenomyosis üvariety of reproductive ages ücysts ≥ 1 cm

Pathogenesis • • • The pathogenesis of adenomyosis is not known. The two major theories Endomyometrial invagination Metaplastic process (Rokitansky-Kuster-Hauser syndrome) Adenomyotic glands differ in expression of key molecules from eutopic endometrial glands Estrogen and progesterone appear Early exposure to estrogen (tamoxifen) increased risk Pituitary protein hormones have roles in the pathogenesis Depression and antidepressant use are increased
Junctional zone • May play a key role in this disease • A region appearing as a dark band on T 2 -weighted MRI • Separates the subendometrial myometrium from the outer myometrium but cannot be identified histologically. • Ultrastructural changes • Differential growth factor expression

Pathogenesis • Similar pathogenesis with myoma üGrowth factor dysregulation üAbnormalities of angiogenesis • Not related with endometriosis although: üEctopic endometrium üCause of pelvic pain

Prevalence and risk factors • Prevalence is uncertain because need pathology of hysterectomy • Prevalence is 20 to 35 percent • Prevalence is up to 65% according clinical diagnosis with imaging • Symptoms are in 40 and 50 years • Younger women are less likely to undergo definitive reproductive surgery. • Dyspareunia is not a typical symptom( In contrast with endometriosis)
Clinical presentation • Heavy menstrual bleeding in 60 percent of women üMay be due to increased endometrial surface üoverexpression of inflammatory mediators in adenomyotic tissue samples • Dysmenorrhea in 25 percent of women due to üBleeding üSwelling of endometrial islands confined by myometrium • Chronic pelvic pain may also occur • Approximately one-third of women are asymptomatic

Evaluation • History, • Pelvic examination, • Imaging. • Laboratory testing may be performed to evaluate for anemia
Pelvic examination • In bimanual pelvic examination, uterus is • mobile, • diffusely enlarged (often referred to as "globular" enlargement), • soft (often referred to as "boggy"). • The uterus may be tender. • Not result in a fixed uterus ( but this may occur with endometriosis, which also often co-occurs with adenomyosis).

Laboratory tests • A urine or serum HCG to exclude pregnancy • A hemoglobin/hematocrit in heavy menstrual bleeding and anemia • Tests to exclude infection may be sent, if pelvic pain is present
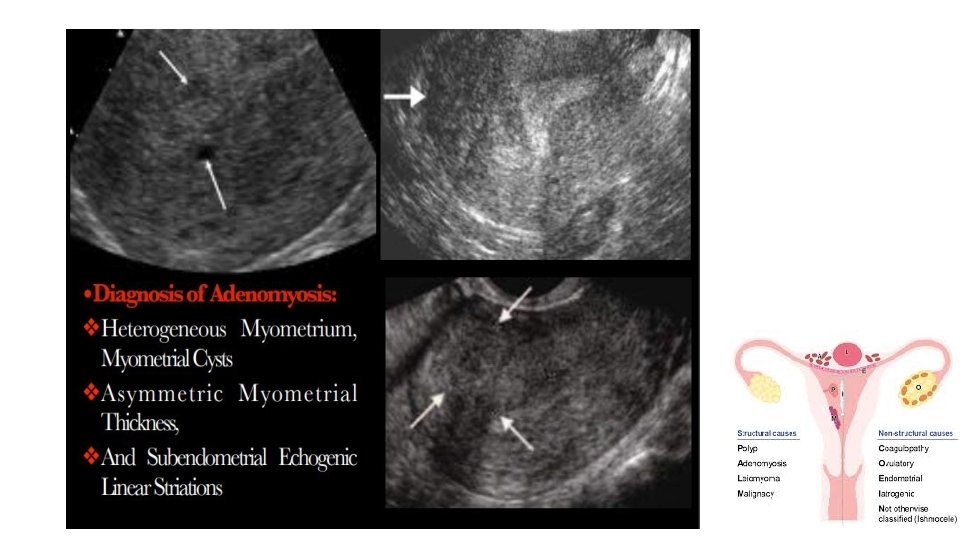
Imaging • Transvaginal ultrasound is the first-line imaging choice • Magnetic resonance imaging (MRI) üTo distinguish diffuse and focal adenomyosis from leiomyomas üTo determine management üPlanning conservative surgery for adenomyosis or fibroids üTo assist in surgical planning.
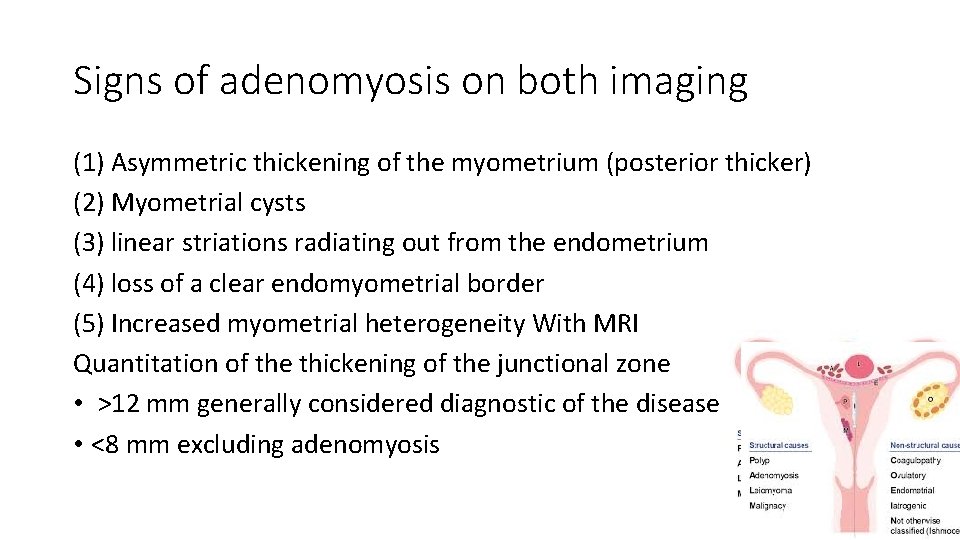
Signs of adenomyosis on both imaging (1) Asymmetric thickening of the myometrium (posterior thicker) (2) Myometrial cysts (3) linear striations radiating out from the endometrium (4) loss of a clear endomyometrial border (5) Increased myometrial heterogeneity With MRI Quantitation of the thickening of the junctional zone • >12 mm generally considered diagnostic of the disease • <8 mm excluding adenomyosis

Biopsy • Endometrial biopsy • Not informative Malignancymust be excluded • Needle biopsy of the myometrium • Not a common practice • Sensitivity of needle biopsy depends upon several factors, including üExtent of disease üNumber of biopsy specimens obtained üSampling site üNeedle gauge üOperator experience

Uterine leiomyomas • Uterine leiomyomas (also referred to as fibroids or myomas) • The most common pelvic tumor in women • They are noncancerous monoclonal tumors • Arising from the smooth muscle cells and fibroblasts of the myometrium. • They arise in reproductive-age women • symptoms of AUB and/or pelvic pain/pressure • Uterine fibroids may also have reproductive effects • (eg, infertility, adverse pregnancy outcomes).

Intramural myomas • (FIGO type 3, 4, 5) ülocated within the uterine wall. üSome fibroids may be transmural and extend from the serosal to the mucosal surface.
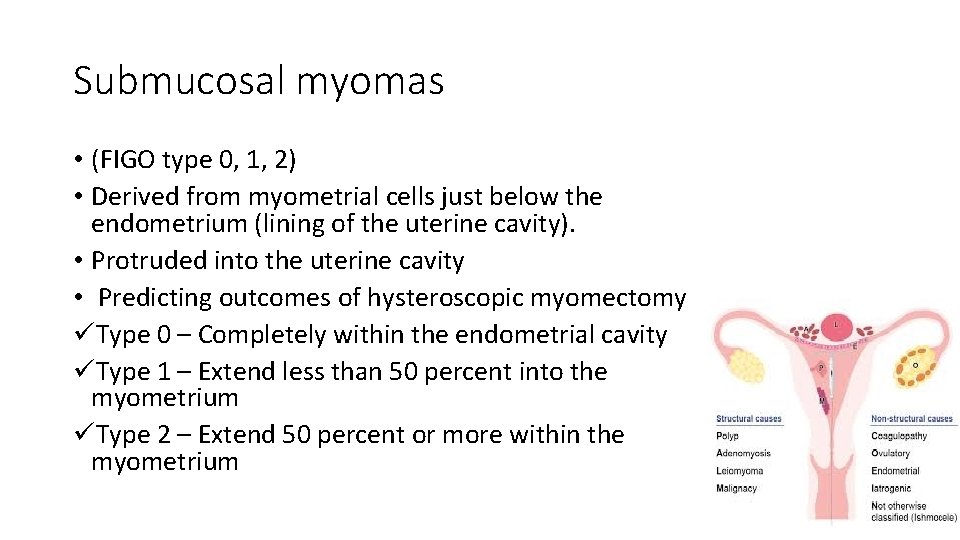
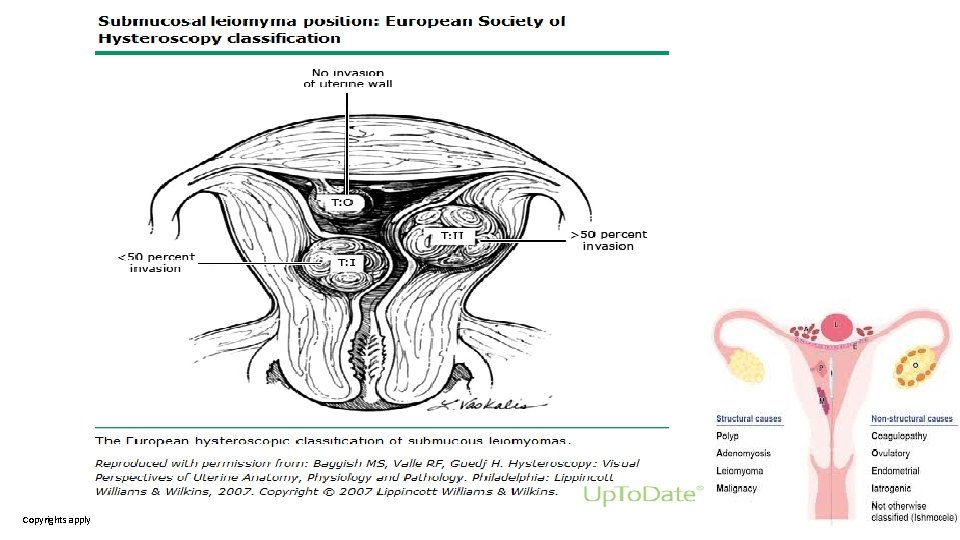
Submucosal myomas • (FIGO type 0, 1, 2) • Derived from myometrial cells just below the endometrium (lining of the uterine cavity). • Protruded into the uterine cavity • Predicting outcomes of hysteroscopic myomectomy üType 0 – Completely within the endometrial cavity üType 1 – Extend less than 50 percent into the myometrium üType 2 – Extend 50 percent or more within the myometrium
Copyrights apply
Subserosal myomas • (FIGO type 6, 7) üOriginate from the myometrium at the serosal surface of the uterus. üBroad or pedunculated base ü May be intraligamentary (ie, extending between the folds of the broad ligament).

Cervical myomas • (FIGO type 8) • Located in the cervix rather than the uterine corpus.

Risk Factor • Race • Two- to threefold greater in black women than in white women • Reproductive and endocrine factors • Parallels the ontogeny and life cycle changes of the reproductive hormones estrogen and progesterone. • Growth of fibroids is responsive to gonadal steroids • Gonadal Horman not necessarily responsible for the genesis of the tumors.
Risk Factor • Parity decreases the chance of fibroid formation. • There is a suggestion that additional pregnancies further decrease the risk • Older age at first birth was also associated with a decreased risk compared with younger age at first birth • A longer interval since last birth with an increased risk
Risk Factor • Early menarche (<10 years old) is associated with an increased risk of developing fibroids. • Increase of estradiol to post-pubertal levels lead to increased fibroid • Obesity • Increasing (BMI) increases fibroids
Risk Factor • Hormonal contraception • Lower dose oral contraceptives is not contraindicated • Other endocrine factors • Prenatal exposure to diethylstilbestrol increased risk of fibroids, • supporting the role of early hormonal exposure in pathogenesis
Risk Factor • Diet • Beef and other reds meats (1. 7 -fold) or ham (1. 3 -fold) increased risk • Green vegetables (0. 5 -fold) and fruit (especially citrus fruit) with a decreased risk • Dietary glycemic index or load are associated with a small increase risk • Dietary vitamin A from animal sources may decreased risk • Vitamin D deficiency or insufficiency may icreased risk

Risk Factor • Alcohol, especially beer, increased risk • Smoking üEarly studies showed that smoking decreased the risk of having fibroids, possibly through the inhibition of aromatase üSubsequent studies have not found an association with fibroids
Risk Factor • Genetics üFamilial predisposition to leiomyomas üSpecific susceptibility genes for fibroids • Other factors üHypertension increased leiomyoma risk. üType 2 diabetes decreased leiomyoma risk
Clinical features • 1 percent of women have fibroids that receive medical attention in a year • The majority are small and asymptomatic • These symptoms are related to the number, size, and location of the tumors. • Myomas can occur as single or multiple tumors and range in size from microscopic to tens of centimeters.
Symptoms • Heavy or prolonged menstrual bleeding • Bulk-related symptoms, such as pelvic pressure and pain • Reproductive dysfunction (ie, infertility or obstetric complications)
• Submucosal myomas • Related to significant heavy menstrual bleeding • Intramural myomas • Heavy or prolonged menstrual bleeding • Subserosal fibroids are not considered a major risk for heavy menstrual bleeding. • Cervical fibroids • Myoma close to the endocervical canal may be related to AUB.
Bulk-related symptoms • Pelvic pressure or pain • Urinary tract or bowel issues • Venous compression • Other pain or discomfort issues • Painful menses • Painful intercourse • Fibroid degeneration or torsion
Infertility or obstetric complications • Infertility üLeiomyomas that distort the uterine cavity üsubmucosal or intramural with an intracavitary component • Adverse pregnancy outcomes üplacental abruption üfetal growth restriction ümalpresentation üpreterm labor and birth).
• Prolapsed fibroid present with ümass übleeding üpossible ulceration or infection • Endocrine effects (With secrete ectopic hormones include): üPolycythemia from autonomous production of erythropoietin üHypercalcemia from autonomous production of parathyroid hormone-related protein üHyperprolactinemia
Physical examination • Abdominal and pelvic examination • Vital signs • Fever, in some women with degenerating fibroids. • Anemia in severe heavy menstrual bleeding • Change in heart rate or blood pressure is rare • Palpation for a pelvic-abdominal mass • The level of the uterine fundus should be noted.
Laboratory testing • Laboratory testing does not have a role in the diagnosis • Hematocrit for anemia • Thyroid-stimulating hormone to rule out concomitant hypothyroidism • A urine or serum human chorionic gonadotropin is ordered if the patient may be pregnant.
Imaging and endoscopy • Pelvic ultrasound is the imaging study of choice • Saline-infused sonogram • Hysteroscopy • Magnetic resonance imaging (MRI) • Computed tomography has little clinical utility in relation to the endometrium or myometrium • Hysterosalpingograms can also sometimes show the distortion of the endometrial cavity
Imaging • Step one: Pelvic ultrasound • Usually as hypoechoic, well-circumscribed round masses, frequently with shadowing; • Cellular fibroids may appear to be more isoechoic, making differentiation from the normal myometrium difficult, or hyperechoic. • Adenomyomas can mimic the appearance of cellular fibroids or multiple small fibroids. • Sarcoma is also difficult to differentiate on imaging.
Imaging • Calcification in a fibroid degenerated. • On plain film as "popcorn" calcifications in the pelvis. • On ultrasound, the calcifications may appear as clumps or rim-like calcifications within a mass • Renal ultrasound for R/O hydronephrosis. • If urinary tract obstruction
Saline infusion sonography • Step two: Evaluate the uterine cavity in women with suspected sub-mucous fibroids or those desiring fertility üSubmucosal lesions (some of which may not be seen on routine ultrasonography) üIntramural myomas that protrude into the cavity and characterizes the extent of protrusion into the endometrial cavity
Hysteroscopy • Visualizing the endometrial cavity. • Submucosal or protruding myometrial fibroids • Can characterize the extent of protrusion. • When the entire fibroid is visualized arising from a pedicle, or has a broad base, the lesion is hysteroscopically classified as intracavitary.
Magnetic resonance imaging Step three: For complex intervention or malignant disease MRI is the most effective modality Visualizing the size and location Can distinguish among leiomyomas, adenomyosis, and adenomyomas. For procedural planning for complicated procedures For women with type 3 through 6 uterine fibroids to know the expected depth into the myometrium for laparoscopy • Predict uterine artery embolization outcome. • Identify features concerning for leiomyosarcoma • • •
Managment • Are submucosal fibroids present? üAUB or infertility, • Are fibroids in one or more than one location? üConsideration all fibroid locations. • Is consistent with bulk-related symptoms? üPelvic pain or pressure is likely to occur only if the uterus is sufficiently enlarged. üIs there urinary symptoms ? anterior fibroid • Is there bowel symptoms? üposterior or left fibroid that puts pressure on the rectum or sigmoid colon. • Is there hydronephrosis? To avoid renal failure.
Cesarean scar defect • A thinning and indentation of the myometrium at the hysterotomy site • Results from inadequate healing of the myometrium at this site. • It has been called by various terms, including niche, isthmocele, and uteroperitoneal fistula. • These defects are more common with increasing numbers of cesarean
Complications of Isthmocele • cesarean scar pregnancy • postmenstrual spotting • pelvic pain, dysmenorrhea • Dyspareunia • uterine rupture • secondary infertility
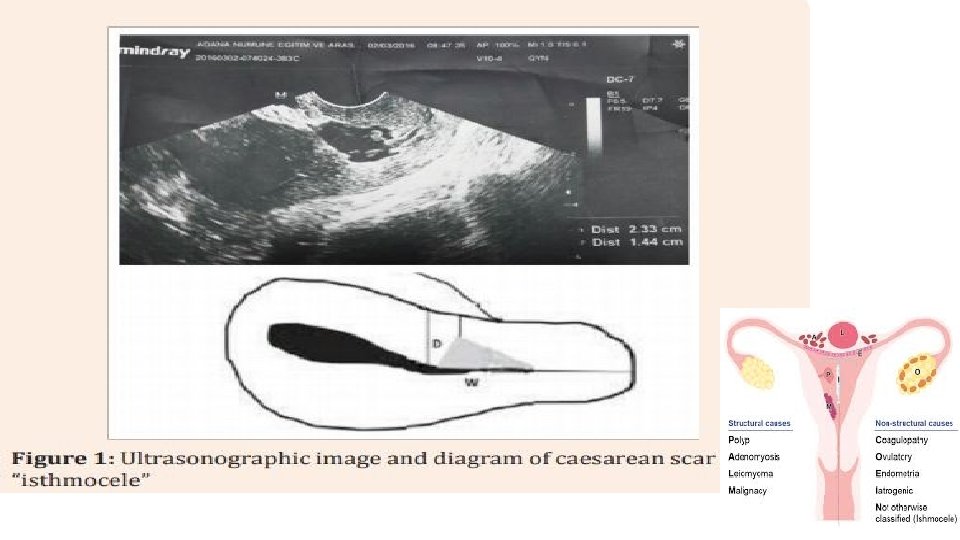
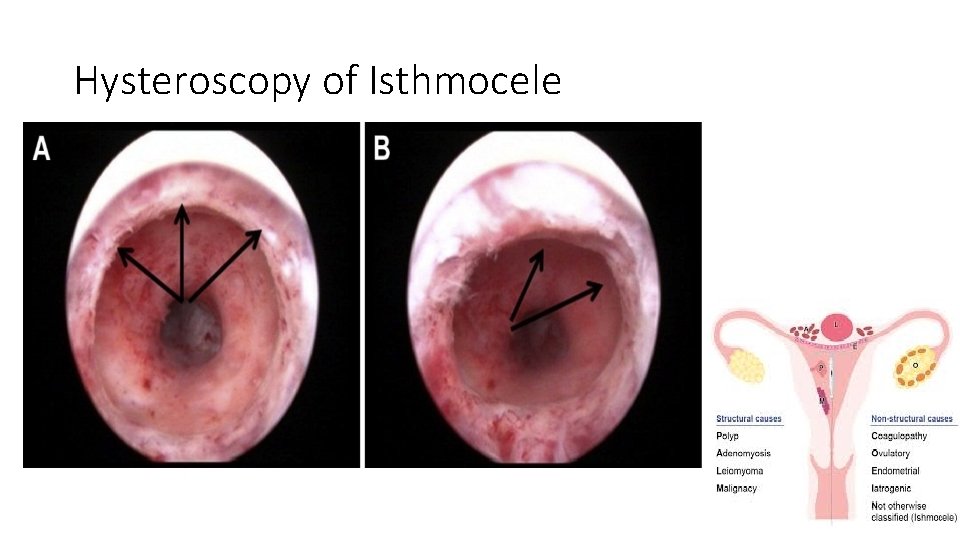
Hysteroscopy of Isthmocele
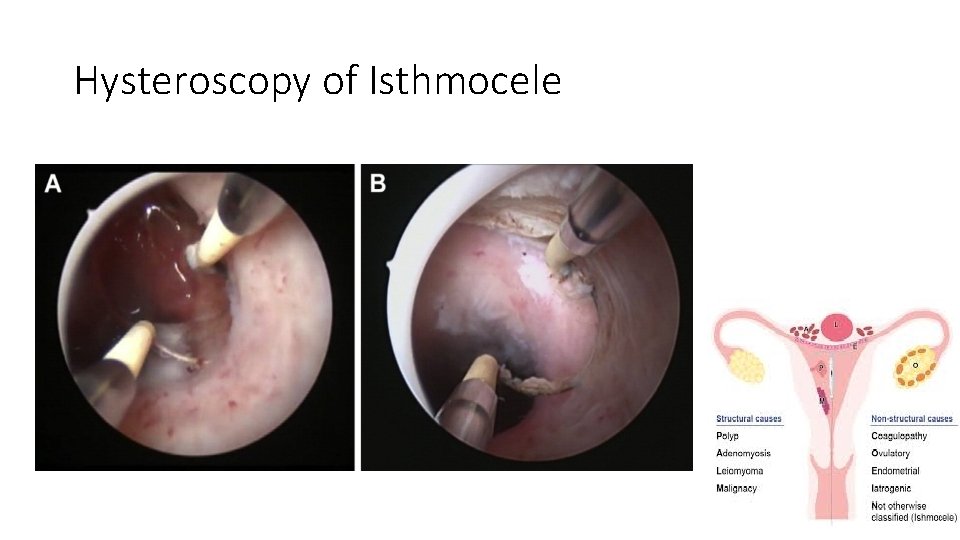
Hysteroscopy of Isthmocele
Endometrial hyperplasia • Non-neoplastic entities üDisordered proliferative endometrium ü Benign hyperplasia üSimple and complex hyperplasias without atypia • Precancerous neoplasms • Endometrial intraepithelial neoplasms [EIN] • Atypical complex hyperplasia • Neoplastic features but without invasion.
• EH frequently results from chronic estrogen stimulation unopposed by the counterbalancing effects of progestrone • Obese women • Chronic ovulatory dysfunction (eg, polycystic ovary syndrome) • Women at increased genetic risk of endometrial cancer (Lynch syndrome). • The majority of women with EH present with abnormal uterine bleeding.

Factors for choosing management • Risk factors for recurrence or progression • Age • EH and endometrial cancer can be observed in women ages <25 • Body mass index >30 kg/m 2. • Desire for fertility. • Contraceptive needs. • Postmenopausal women with endometrial thickness • On TVS ET≥ 20 mm have a greater risk for concomitant endometrial cancer
Pathology • Proliferative endometrium • Nonatypical hyperplasia/benign endometrial hyperplasia • Simple hyperplasia without atypia/benign endometrial hyperplasia/disordered proliferative endometrium • Complex hyperplasia without atypia/benign endometrial hyperplasia/gland crowding • Atypical hyperplasia/EIN • endometrial carcinoma
Endometrial carcinoma • Endometrioid carcinoma is the most common • Present at an early stage with AUB • Other histologic types of endometrial carcinoma (eg, serous, clear cell) as poor prognosis. • The incidence peaks between ages 60 and 70 years • under age 50 with chronic anovulation and/or obesity. • Histologic diagnosis with endometrial biopsy, endometrial curettage, or hysterectomy specimen.

- Slides: 69