Ab Initio Methods for Protein Structure Prediction CS
Ab Initio Methods for Protein Structure Prediction CS 882 Presentation, by Shuai C. , Li

Motivation n homology modeling n No knowledge about the physical nature of the protein folding and stability. n ab-initio methods can n augment fold-recognition and homology (refinement, large loops, side chains). n it can ease experimental structure determination. n It can find new folds

Ab Initio Methods n Ab initio: “From the beginning”. n Assumption n All the information about the structure of a protein is contained in its sequence of amino acids. n The structure that a (globular) protein folds into is the structure with the lowest free energy. n The native structure is contained in the search space n Finding native-like conformations require n A scoring function (potential). n A search strategy.

ab-initio protein structure prediction n Optimization problem n n Define some initial model. Define a function mapping structures to numerical values (the lower the better). Solve the computational problem of finding the global minimum. Simulation of the actual folding process n n Build an accurate initial model (including energy and forces). Accurately simulate the dynamics of the system. The native structure will emerge. No hope due to large search space
Energy Minimization (Theory) n Treat Protein molecule as a set of balls (with mass) connected by rigid rods and springs n Rods and springs have empirically determined force constants n Allows one to treat atomic-scale motions in proteins as classical physics problems
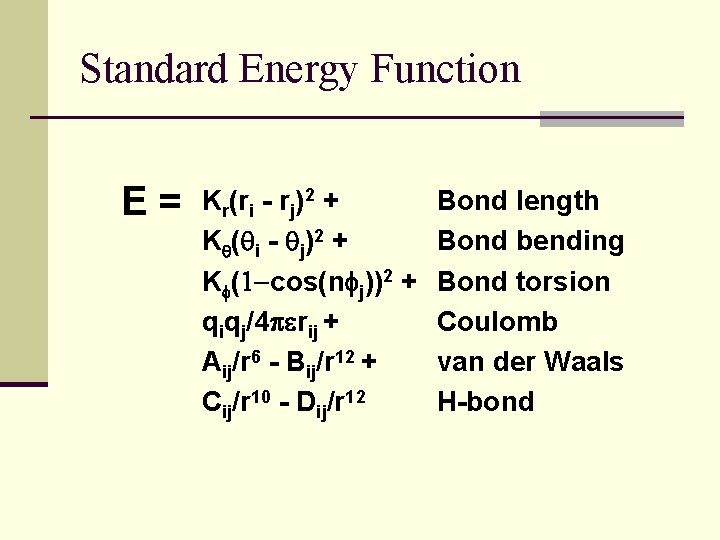
Standard Energy Function E= Kr(ri - rj)2 + Kq(qi - qj)2 + Kf(1 -cos(nfj))2 + qiqj/4 perij + Aij/r 6 - Bij/r 12 + Cij/r 10 - Dij/r 12 Bond length Bond bending Bond torsion Coulomb van der Waals H-bond
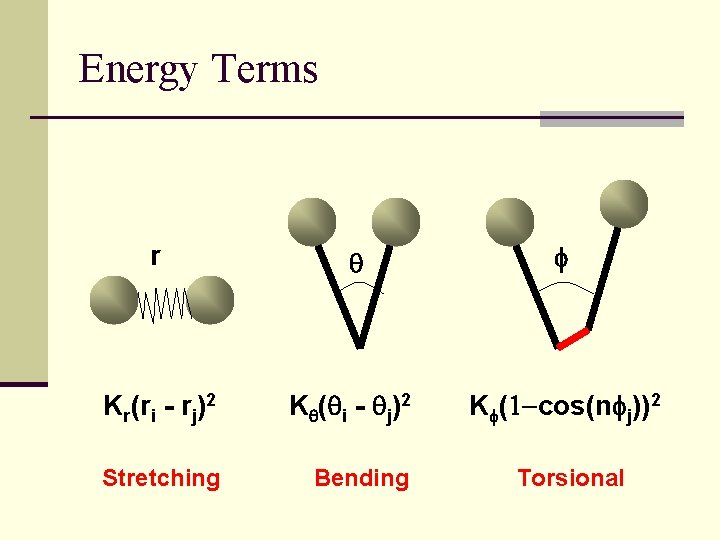
Energy Terms r q f Kr(ri - rj)2 Kq(qi - qj)2 Kf(1 -cos(nfj))2 Stretching Bending Torsional
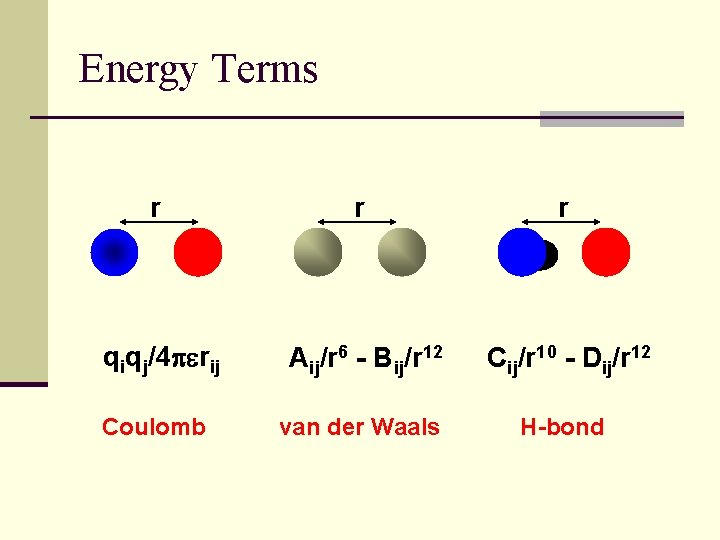
Energy Terms r r r qiqj/4 perij Aij/r 6 - Bij/r 12 Cij/r 10 - Dij/r 12 van der Waals H-bond Coulomb
Reduced complexity models n No side chains n sometimes no main chain atoms either n Or represent the side chain with C n Reduced degrees of freedom n On-or off-lattice n Generally have an environment -based score and a knowledge-based residue-residue interaction term n Sometimes used as first step to prune the enormous conformational space, then resolution is increased for later fine-tuning
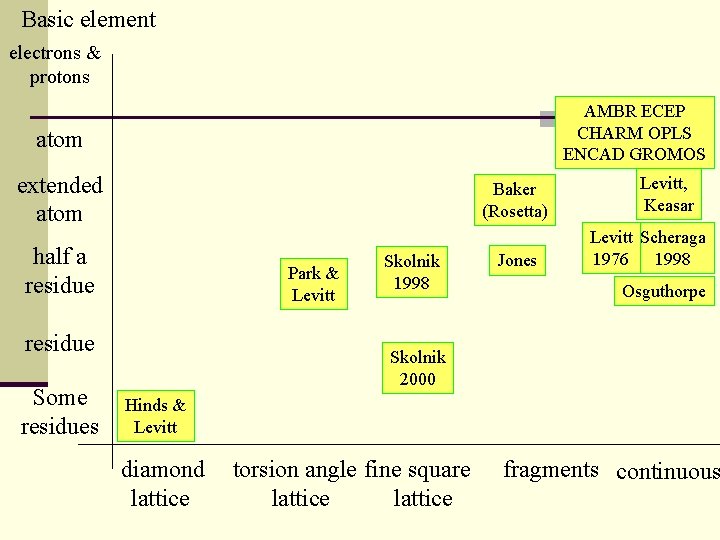
Basic element electrons & protons AMBR ECEP CHARM OPLS ENCAD GROMOS atom extended atom Baker (Rosetta) half a residue Park & Levitt residue Some residues Skolnik 1998 Jones Levitt, Keasar Levitt Scheraga 1976 1998 Osguthorpe Skolnik 2000 Hinds & Levitt diamond lattice torsion angle fine square lattice fragments continuous
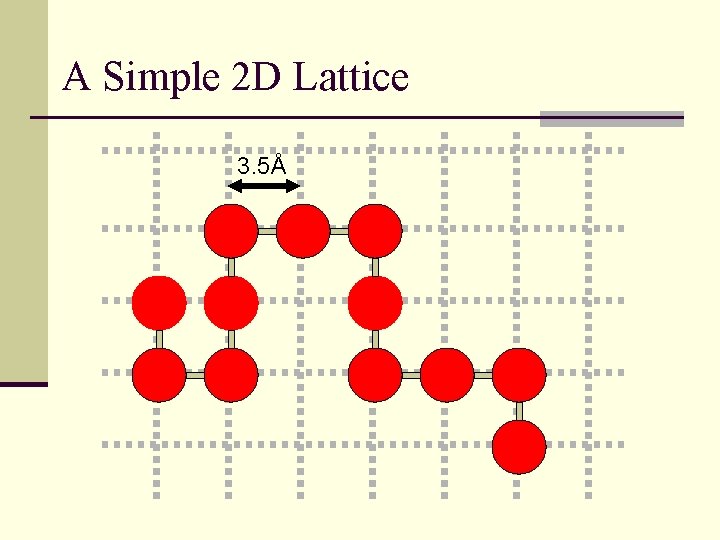
A Simple 2 D Lattice 3. 5Å
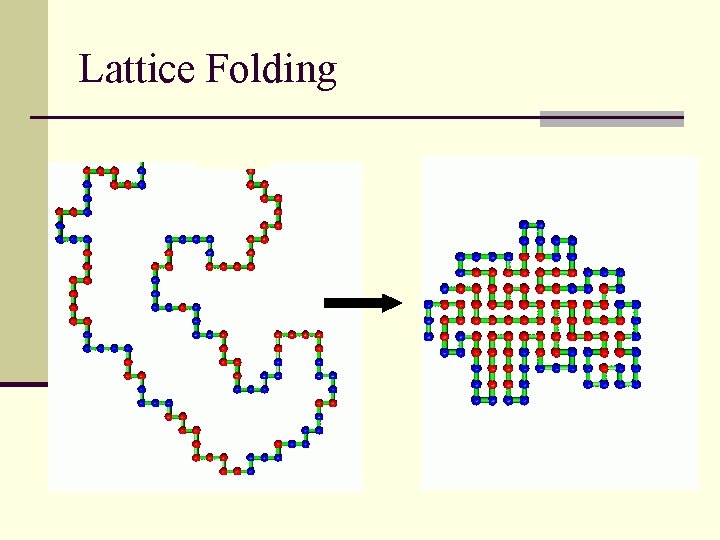
Lattice Folding

Lattice Algorithm n Build a “n x m” matrix (a 2 D array) n Choose an arbitrary point as your N terminal residue n n (start residue) Add or subtract “ 1” from the x or y position of the start residue Check to see if the new point (residue) is off the lattice or is already occupied Evaluate the energy Go to step 3) and repeat until done
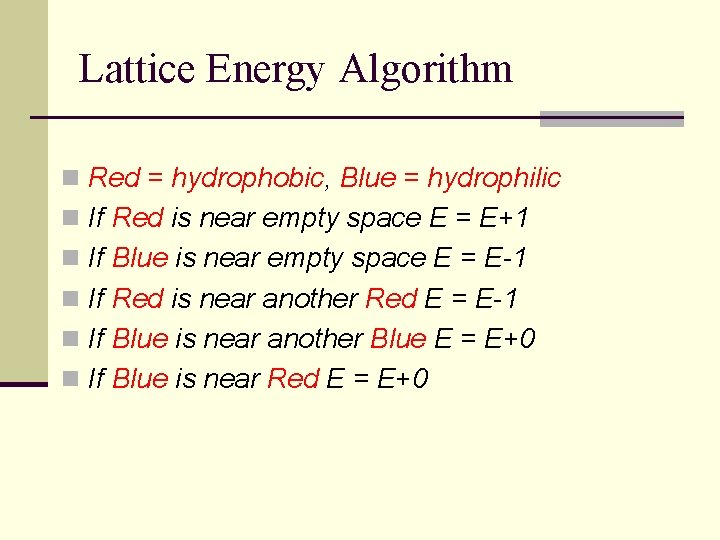
Lattice Energy Algorithm n Red = hydrophobic, Blue = hydrophilic n If Red is near empty space E = E+1 n If Blue is near empty space E = E-1 n If Red is near another Red E = E-1 n If Blue is near another Blue E = E+0 n If Blue is near Red E = E+0
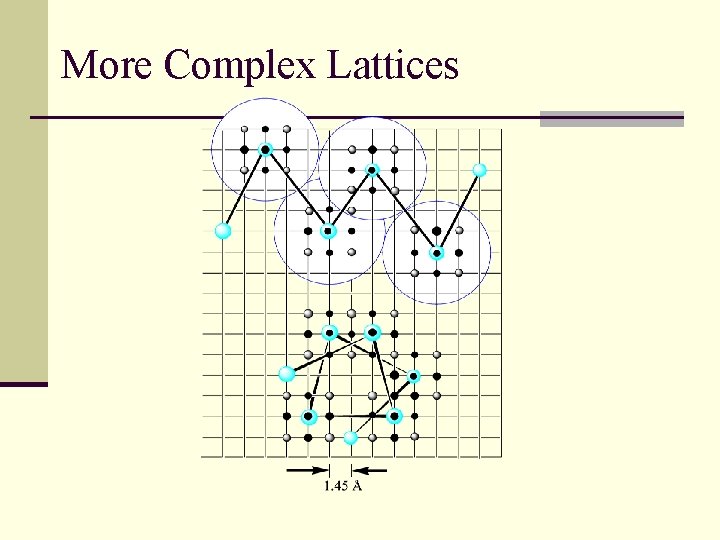
More Complex Lattices
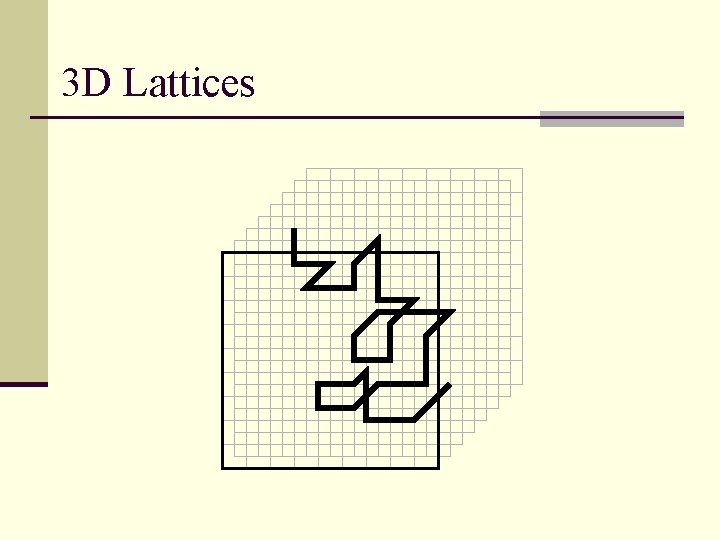
3 D Lattices
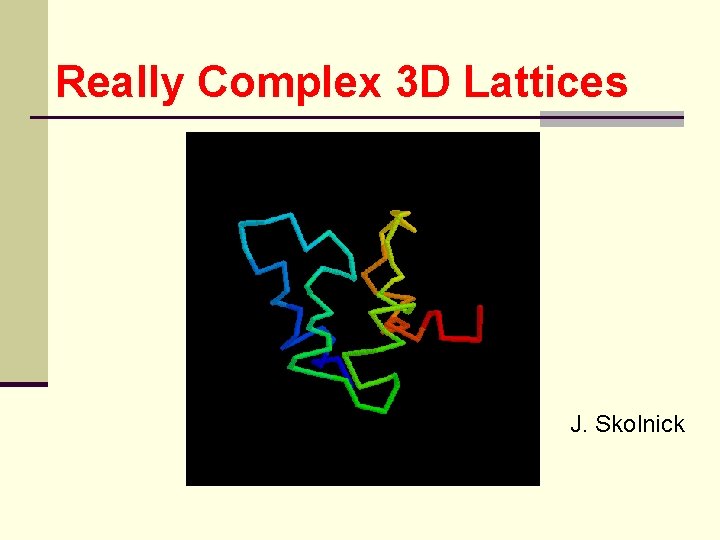
Really Complex 3 D Lattices J. Skolnick
Lattice Methods Advantages n Easiest and quickest way Disadvantages n At best, only an to build a polypeptide approximation to the real thing n More complex lattices allow reasonably accurate n Does not allow accurate representation constructs n Complex lattices are as “costly” as the real thing
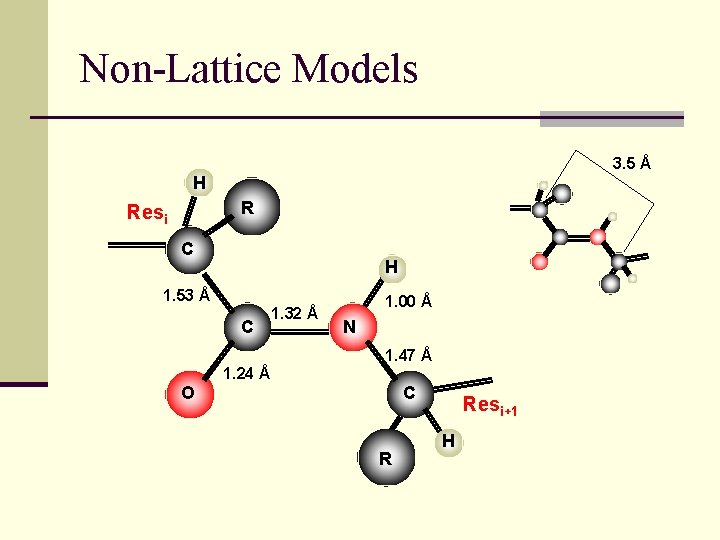
Non-Lattice Models 3. 5 Å H R Resi C H 1. 53 Å C 1. 32 Å 1. 00 Å N 1. 47 Å 1. 24 Å O C R Resi+1 H
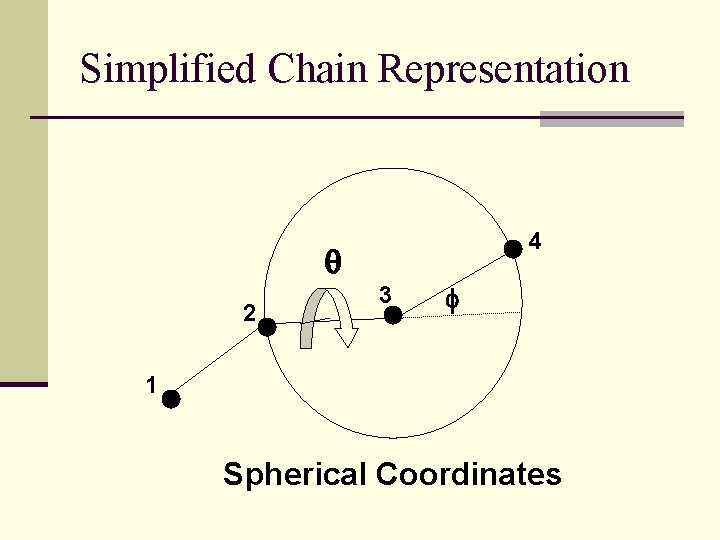
Simplified Chain Representation 4 q 2 3 f 1 Spherical Coordinates
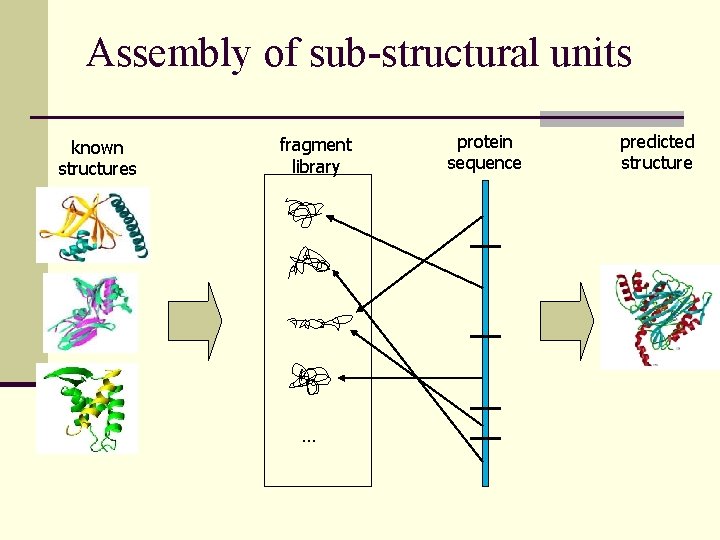
Assembly of sub-structural units known structures fragment library … protein sequence predicted structure

Structure Prediction with Rosetta n Select fragments consistent with local sequence preferences n Assemble fragments into models with nativelike global properties n Identify the best model from the population of decoys

Modelling Protein sequence n Model each candidate local structure as a node
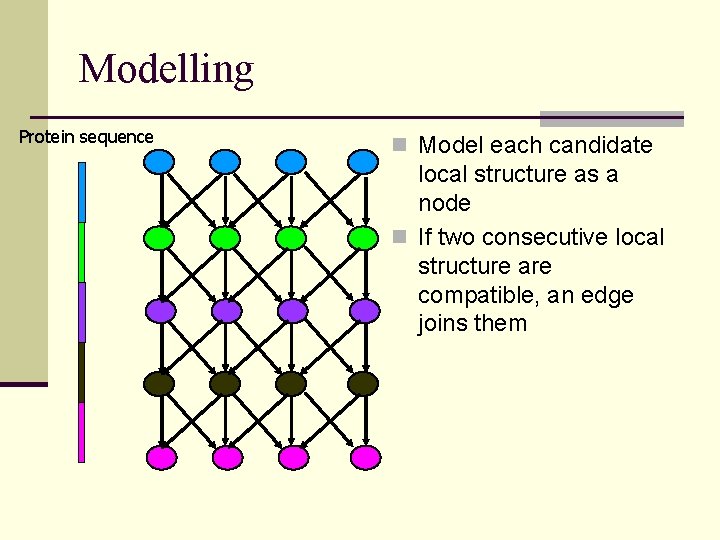
Modelling Protein sequence n Model each candidate local structure as a node n If two consecutive local structure are compatible, an edge joins them
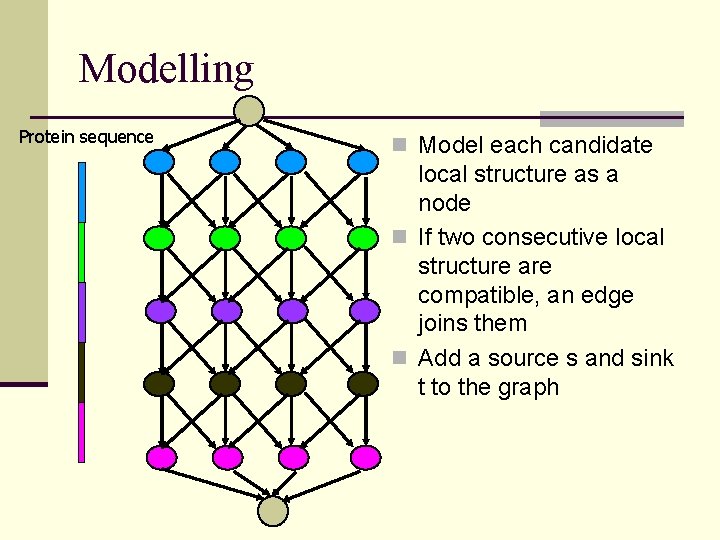
Modelling Protein sequence n Model each candidate local structure as a node n If two consecutive local structure are compatible, an edge joins them n Add a source s and sink t to the graph
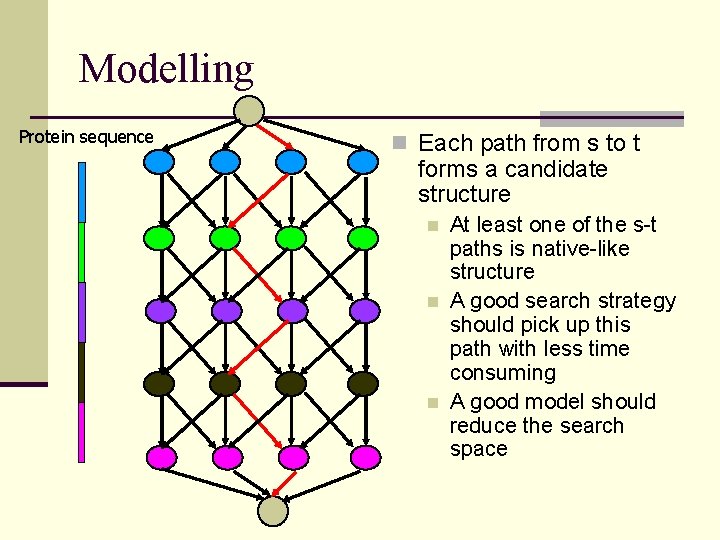
Modelling Protein sequence n Each path from s to t forms a candidate structure n n n At least one of the s-t paths is native-like structure A good search strategy should pick up this path with less time consuming A good model should reduce the search space
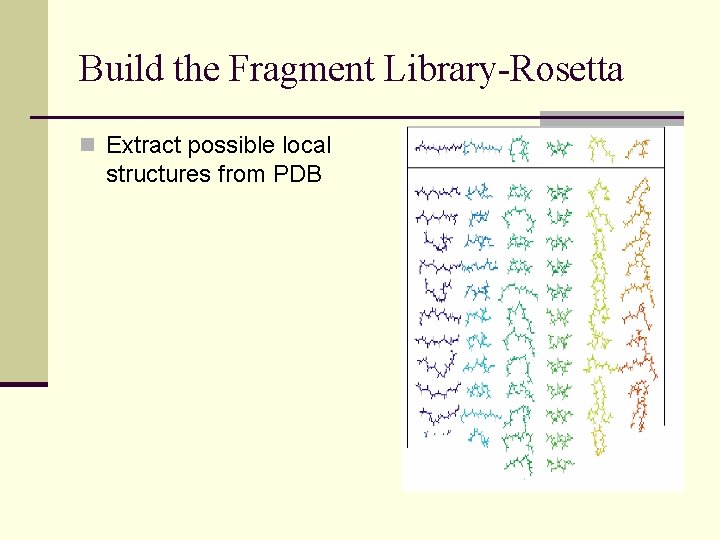
Build the Fragment Library-Rosetta n Extract possible local structures from PDB
Generate the Fragment Library n Select PDB template n Select Sequence Families n Each Family has a single known structure (family) n Has no more than 25% sequence identity between any two sequence n Clustering the fragments n Generate all the fragments from the selected families n
Find Local Structures n Given a subsequence, a local structure to be identified n n n Represent each subsequence with a vector n V={v 1, v 2, …, vk} n eg: V as a 20*l matrix, with the (i, j)-th entry represent the frequency of amino acid j occurs at position j Represent each substructure with a vector n V’={v 1’, v 2’, …, vk’ } n eg: V as a 20*l matrix, with the (i, j)-th entry represent the frequency of amino acid j occurs at position j Rank the structure according to: n i|vi-vi’| n This implies that the entries of the vectors are independent.
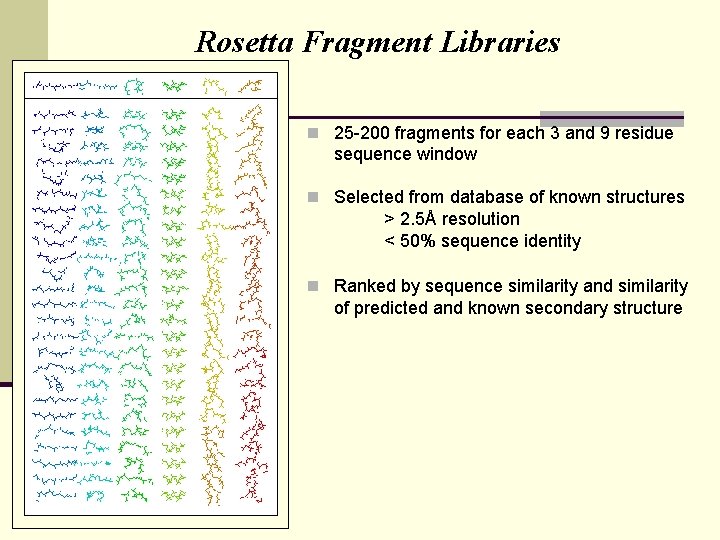
Rosetta Fragment Libraries n 25 -200 fragments for each 3 and 9 residue sequence window n Selected from database of known structures > 2. 5Å resolution < 50% sequence identity n Ranked by sequence similarity and similarity of predicted and known secondary structure

Search Strategy n Reduce the Search Space n Design Better Search Strategies

Scoring Function n Ideal energy function n Has a clear minimum in the native structure. n Has a clear path towards the minimum. n Global optimization algorithm should find the native structure.
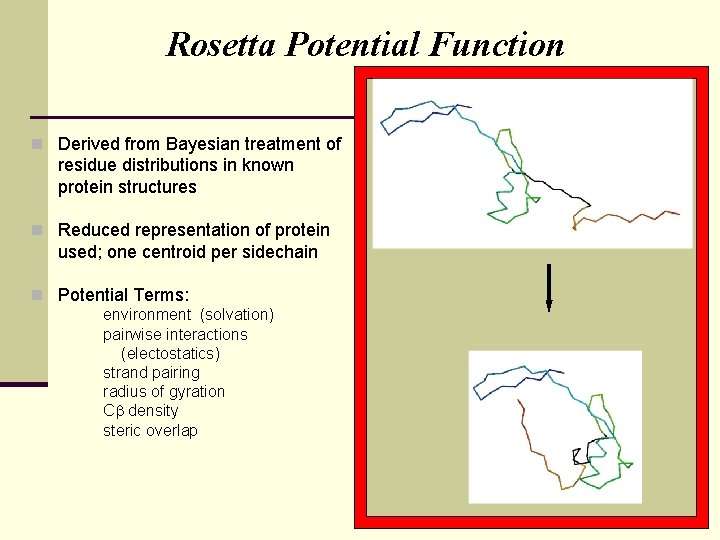
Rosetta Potential Function n Derived from Bayesian treatment of residue distributions in known protein structures n Reduced representation of protein used; one centroid per sidechain n Potential Terms: environment (solvation) pairwise interactions (electostatics) strand pairing radius of gyration C density steric overlap
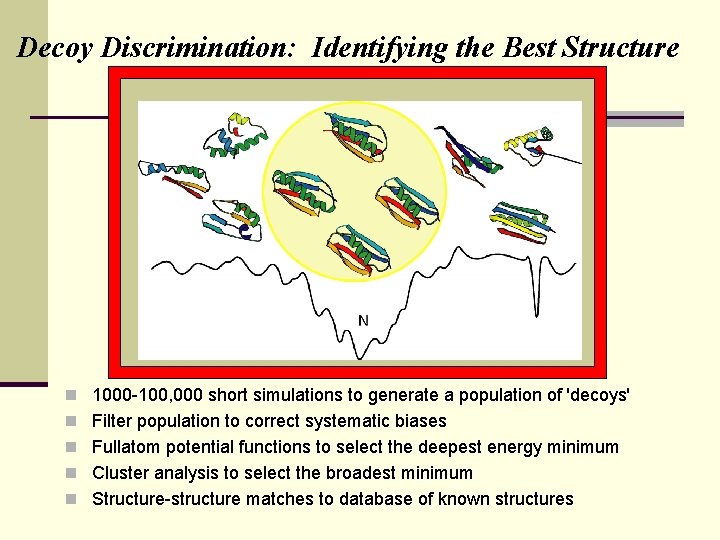
Decoy Discrimination: Identifying the Best Structure n 1000 -100, 000 short simulations to generate a population of 'decoys' n Filter population to correct systematic biases n Fullatom potential functions to select the deepest energy minimum n Cluster analysis to select the broadest minimum n Structure-structure matches to database of known structures
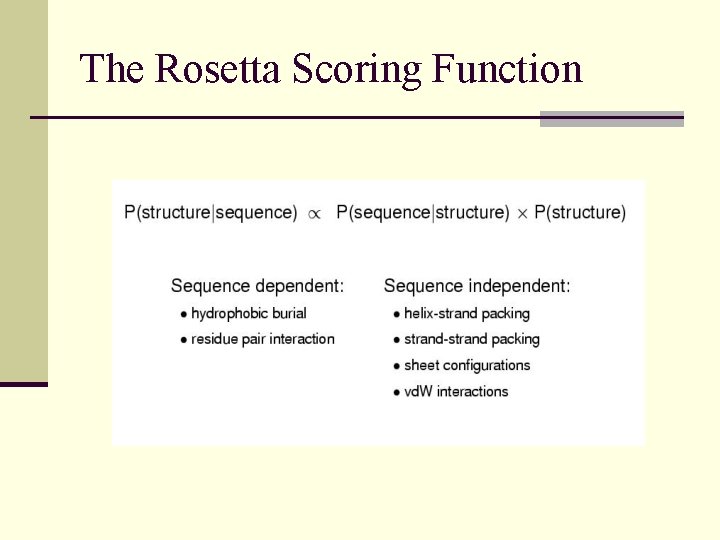
The Rosetta Scoring Function
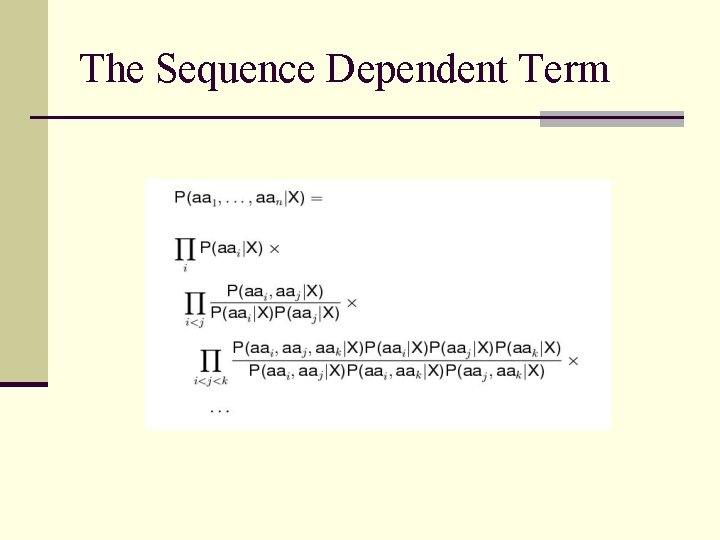
The Sequence Dependent Term
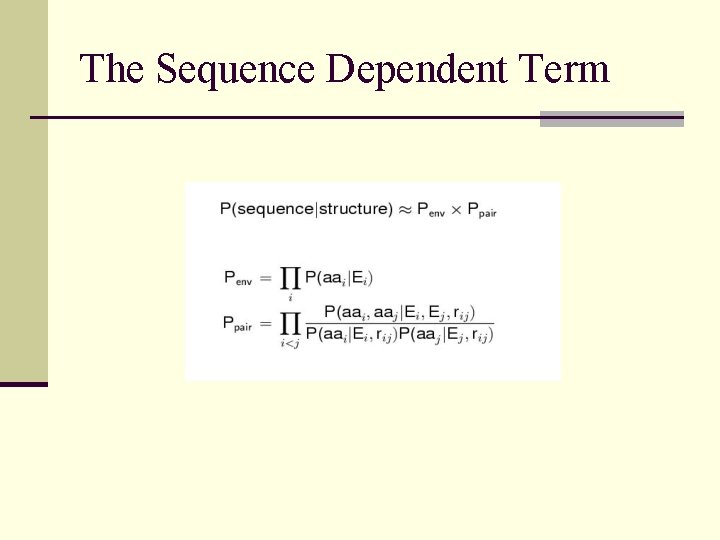
The Sequence Dependent Term
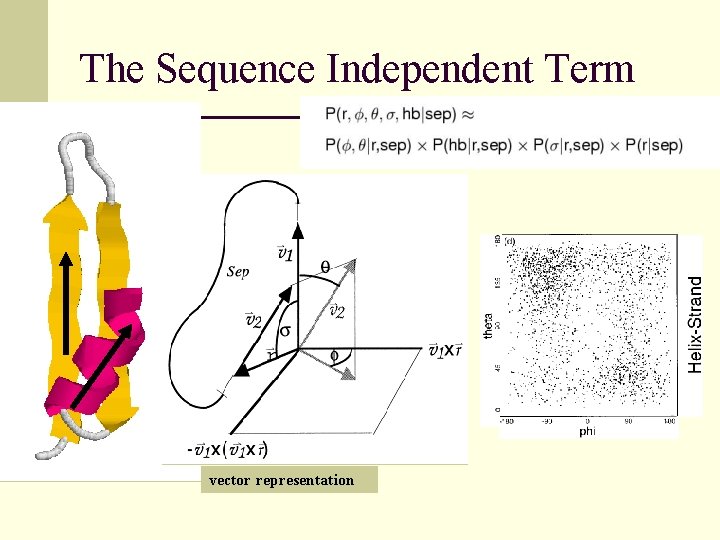
The Sequence Independent Term vector representation
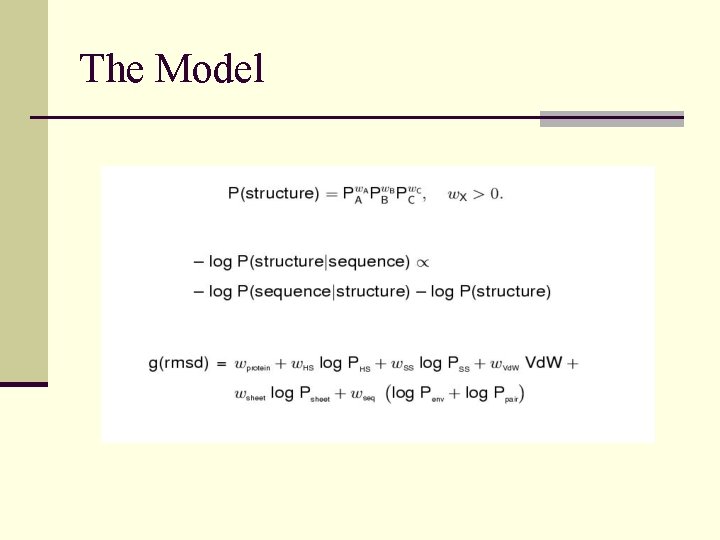
The Model

Search Strategy n Requirement n Identify the native structure easily n Filter out those non-native ones n Eliminate the non-native candidates as early as possible n Jumping out from the local minimum n No repetitions n … n Search Strategies n Taboo search, simulated annealing, genetic algorithms, multi-agent, … n
ROSETTA search algorithm Monte Carlo/Simulated Annealing n Structures are assembled from fragments by: n Begin with a fully extended chain n Randomly replace the conformation of one 9 residue segment with the conformation of one of its neighbors in the library n Evaluate the move: Accept or reject based on an energy function n Make another random move, tabu list is built to forbidden some local minimums n After a prescribed number of cycles, switch to 3 -residue fragment moves

A Filter for Bad -Sheets Many decoys do not have proper sheets. Filtering those out seems to enhance the rmsd distribution in the decoy set. Bad features we see in decoys include: No strands, n Single strands, n Too many neighbors, n Single strand in sheets, n Bad dot-product, n False sheet type (barrel), n
ROSETTA Obstacles & Enhancements n generate lots of unrealistic decoys n Filter based on contact order n quality of β-sheets n poor packing n large search space n Bias fragment picking by predicted secondary structure, faster computational algorithms n low confidence in the result n – Fold many homologs of the target, cluster the answers, report the cluster with highest occupancy

Our Works n Build Fragment Libraries (on the way) n More comprehensive library n Better method to pick up local structures n More accurate energy functions to identify native structures (on the way, joint work with Xin, Gao, and Dongbo, Bu) n Systematic ways to build a promising energy function n Better search strategies n Just have some initial ideas.
- Slides: 44