A very elementary approach to Quantum mechanics There

- Slides: 11

A very elementary approach to Quantum mechanics „There was a time when newspapers said that only twelve men understood theory of relativity. I do not believe that there ever was such a time. . . On the other hand, I think it is safe to say that no one understands quantum mechanics“ R. P. Feynman The Character of Physical Law (1967) let´s approach some aspects of qm anyway

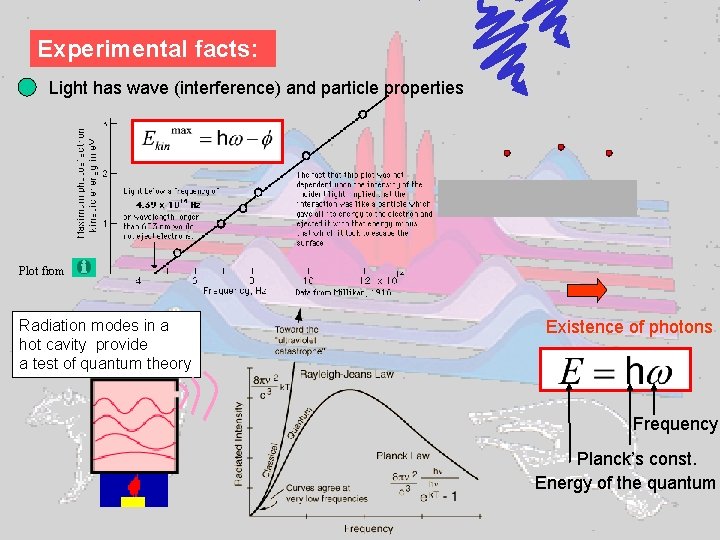
Experimental facts: Light has wave (interference) and particle properties Plot from Radiation modes in a hot cavity provide a test of quantum theory Existence of photons Frequency Planck’s const. Energy of the quantum

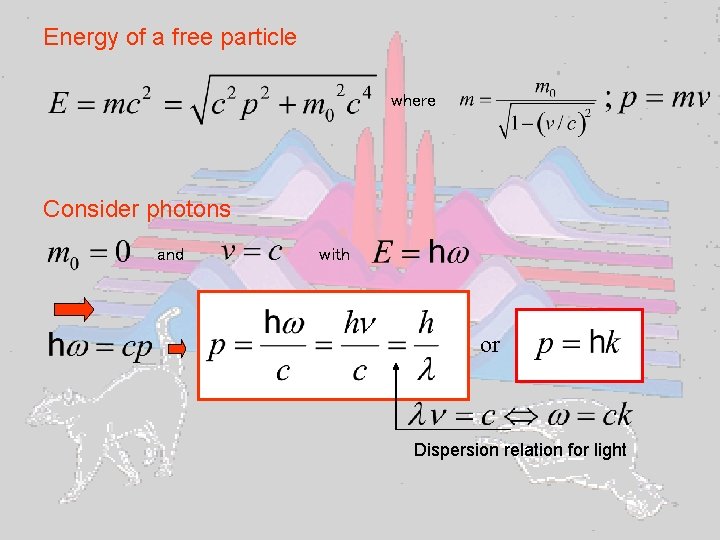
Energy of a free particle where Consider photons and with or Dispersion relation for light

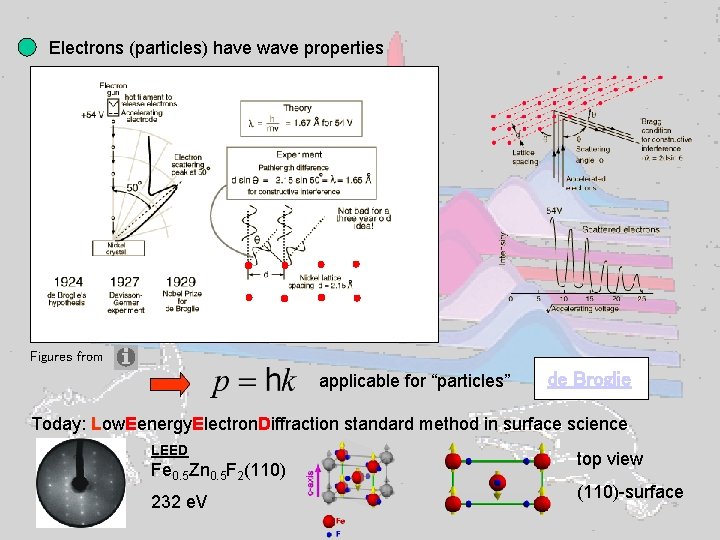
Electrons (particles) have wave properties Figures from applicable for “particles” de Broglie Today: Low. Eenergy. Electron. Diffraction standard method in surface science LEED Fe 0. 5 Zn 0. 5 F 2(110) 232 e. V top view (110)-surface

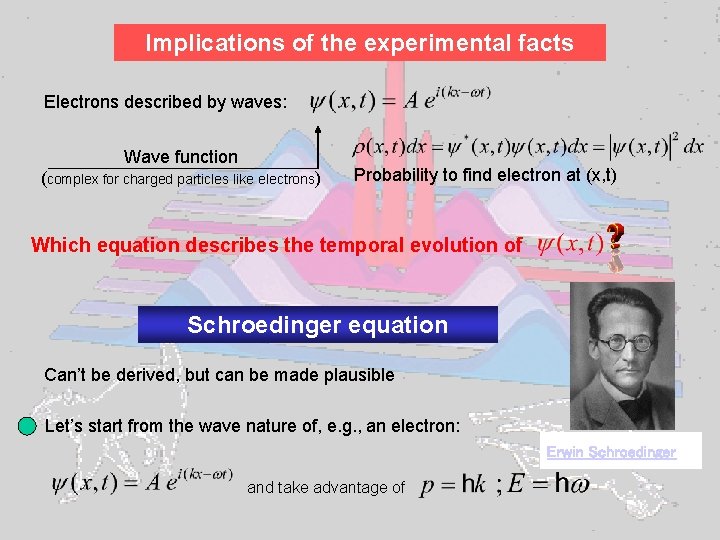
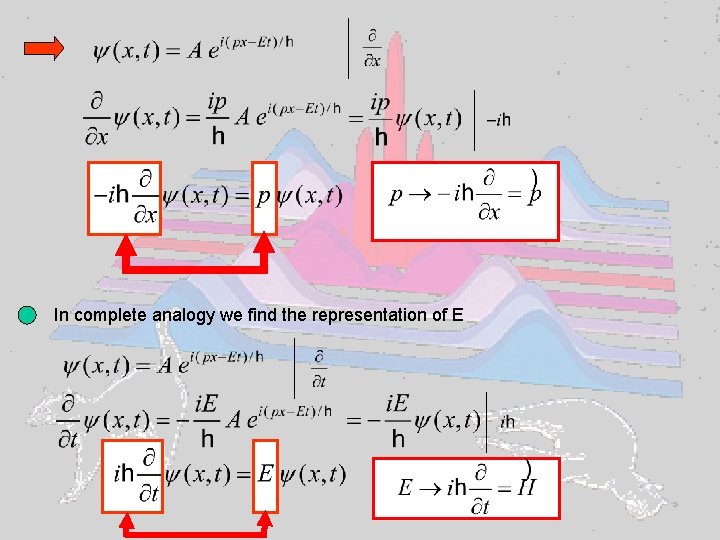
Implications of the experimental facts Electrons described by waves: Wave function (complex for charged particles like electrons) Probability to find electron at (x, t) Which equation describes the temporal evolution of Schroedinger equation Can’t be derived, but can be made plausible Let’s start from the wave nature of, e. g. , an electron: Erwin Schroedinger and take advantage of

In complete analogy we find the representation of E

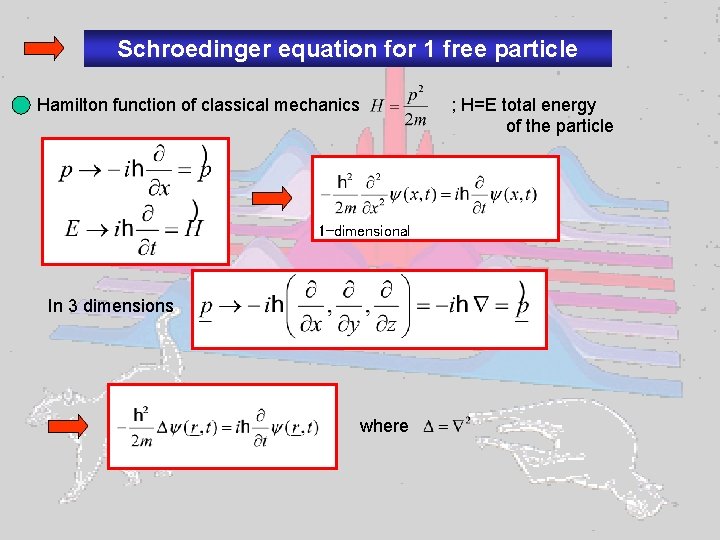
Schroedinger equation for 1 free particle Hamilton function of classical mechanics 1 -dimensional In 3 dimensions where ; H=E total energy of the particle

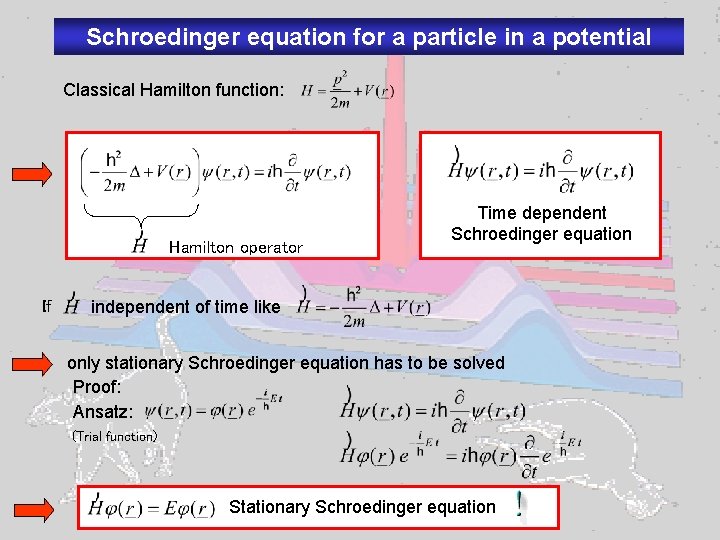
Schroedinger equation for a particle in a potential Classical Hamilton function: Hamilton operator If Time dependent Schroedinger equation independent of time like only stationary Schroedinger equation has to be solved Proof: Ansatz: (Trial function) Stationary Schroedinger equation

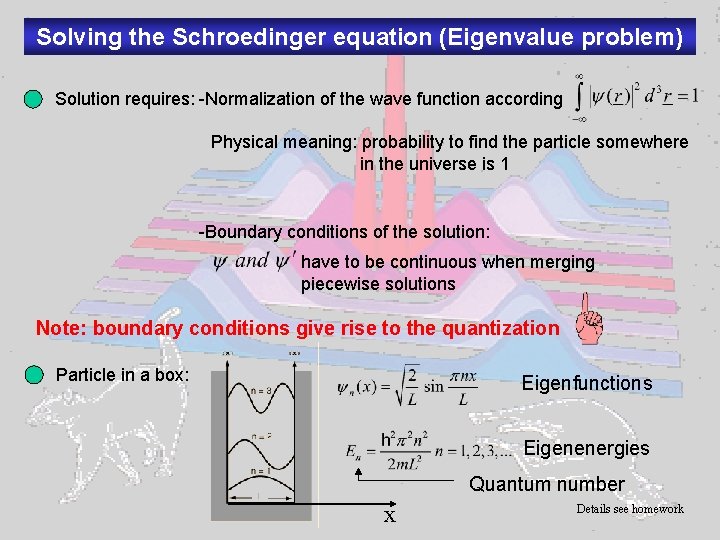
Solving the Schroedinger equation (Eigenvalue problem) Solution requires: -Normalization of the wave function according Physical meaning: probability to find the particle somewhere in the universe is 1 -Boundary conditions of the solution: have to be continuous when merging piecewise solutions Note: boundary conditions give rise to the quantization Particle in a box: Eigenfunctions Eigenenergies Quantum number x Details see homework

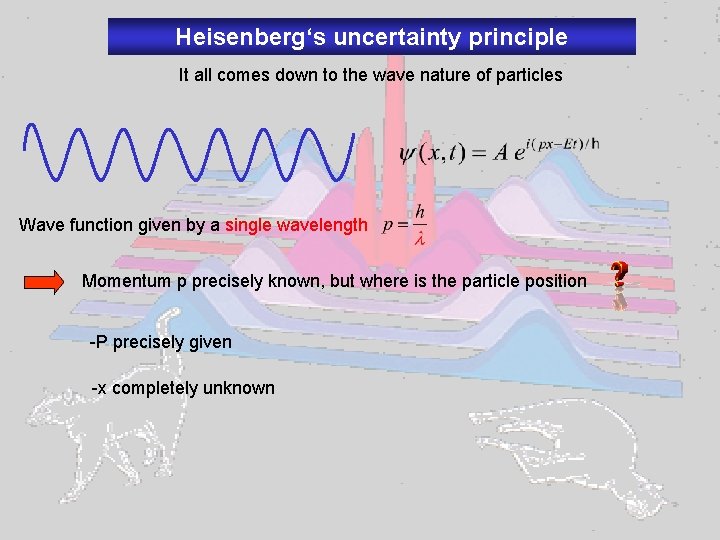
Heisenberg‘s uncertainty principle It all comes down to the wave nature of particles Wave function given by a single wavelength Momentum p precisely known, but where is the particle position -P precisely given -x completely unknown

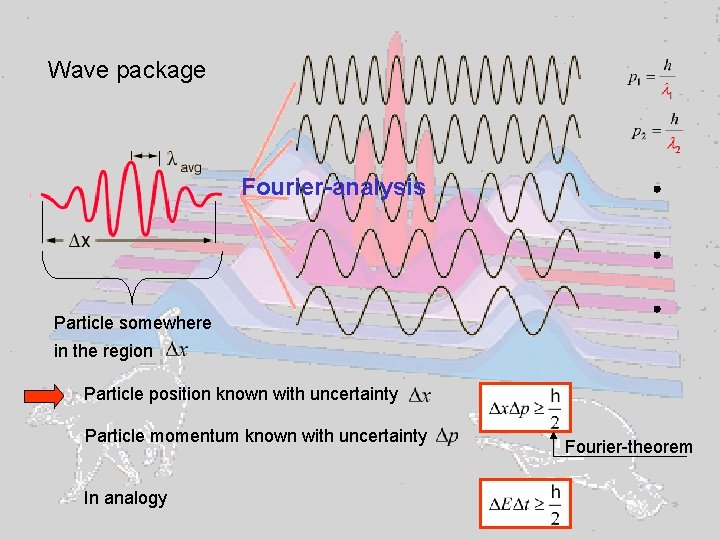
Wave package Fourier-analysis Particle somewhere in the region Particle position known with uncertainty Particle momentum known with uncertainty In analogy Fourier-theorem