A typical type of heat transfer problem which
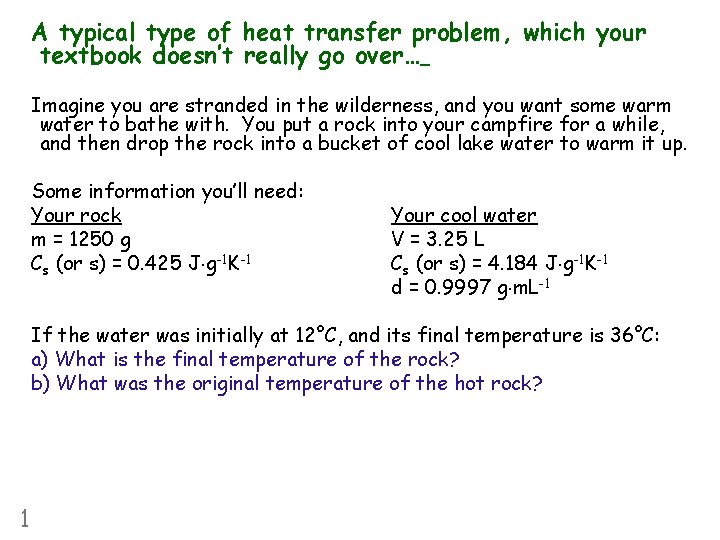
A typical type of heat transfer problem, which your textbook doesn’t really go over… Imagine you are stranded in the wilderness, and you want some warm water to bathe with. You put a rock into your campfire for a while, and then drop the rock into a bucket of cool lake water to warm it up. Some information you’ll need: Your rock m = 1250 g Cs (or s) = 0. 425 J g-1 K-1 Your cool water V = 3. 25 L Cs (or s) = 4. 184 J g-1 K-1 d = 0. 9997 g m. L-1 If the water was initially at 12°C, and its final temperature is 36°C: a) What is the final temperature of the rock? b) What was the original temperature of the hot rock? 1
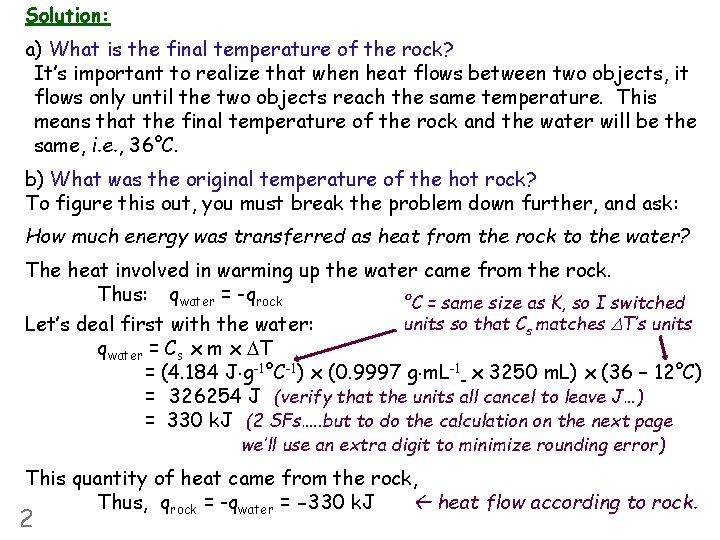
Solution: a) What is the final temperature of the rock? It’s important to realize that when heat flows between two objects, it flows only until the two objects reach the same temperature. This means that the final temperature of the rock and the water will be the same, i. e. , 36°C. b) What was the original temperature of the hot rock? To figure this out, you must break the problem down further, and ask: How much energy was transferred as heat from the rock to the water? The heat involved in warming up the water came from the rock. Thus: qwater = -qrock °C = same size as K, so I switched units so that Cs matches DT’s units Let’s deal first with the water: qwater = Cs x m x DT = (4. 184 J g-1°C-1) x (0. 9997 g m. L-1 x 3250 m. L) x (36 – 12°C) = 326254 J (verify that the units all cancel to leave J…) = 330 k. J (2 SFs…. . but to do the calculation on the next page we’ll use an extra digit to minimize rounding error) This quantity of heat came from the rock, Thus, qrock = -qwater = -330 k. J heat flow according to rock. 2
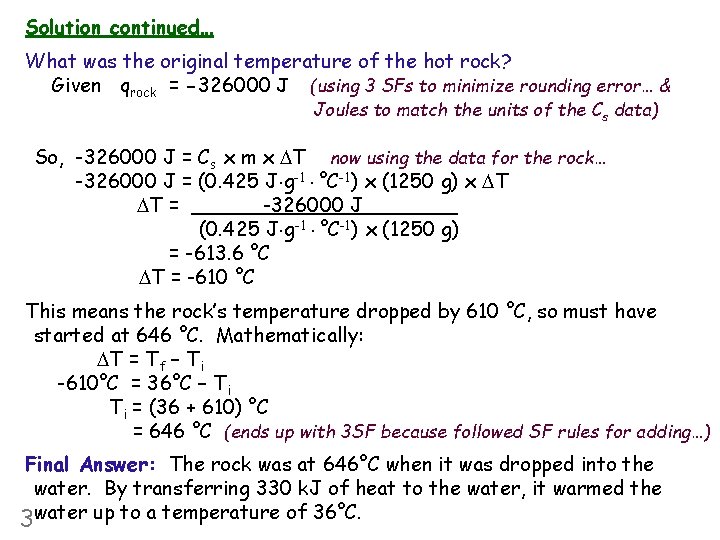
Solution continued… What was the original temperature of the hot rock? Given qrock = -326000 J (using 3 SFs to minimize rounding error… & Joules to match the units of the Cs data) So, -326000 J = Cs x m x DT now using the data for the rock… -326000 J = (0. 425 J g-1 °C-1) x (1250 g) x DT DT = -326000 J (0. 425 J g-1 °C-1) x (1250 g) = -613. 6 °C DT = -610 °C This means the rock’s temperature dropped by 610 °C, so must have started at 646 °C. Mathematically: DT = Tf – Ti -610°C = 36°C – Ti Ti = (36 + 610) °C = 646 °C (ends up with 3 SF because followed SF rules for adding…) Final Answer: The rock was at 646°C when it was dropped into the water. By transferring 330 k. J of heat to the water, it warmed the 3 water up to a temperature of 36°C.
- Slides: 3