A Test of Different Cryopreservation Methods on Members

A Test of Different Cryopreservation Methods on Members of the Nowakoskiella Clade Sharon E. Mozley Chytrid PEET Workshop March 30, 2001 University of Georgia

What is Cryopreservation? Cryopreservation—A technique for freezing tissue or cells to preserve for use at a later date. Cells are usually stored at ultra-low temperatures below -1390 C. Why do it? To Freeze Cultures for storage thus “freeing” us from the bimonthly task of culture transfers with all of the associated risks, i. e. contamination, loss of culture, and mislabeling.

How does Cryopreservation Work? Cryopreservation works by slowing down the cell’s metabolism to the point that no further biochemical reactions can occur. Halting the cell’s metabolism is achieved by lowering the temperature until all the water inside the cell is frozen or removed by osmosis to the outside of the cell. Unfortunately, freezing cells is not a problem-free process. The rate of freezing has to be slow enough to prevent cellular damage due to intracellular ice formation because of the phenomena of supercooling and fast enough to keep the cells from shrinking due to exposure to a hypertonic environment (for a review of Osmosis and Tonicity check out http: //faculty. nl. edu/jste/osmosis. htm. ) So how do we deal with the problems of supercooling and hypertonic environments? --------------------------------------->

We Use Cryoprotectants! Cryoprotectants protect the cells from stresses encountered during freezing by making the concentration of solutes the same inside the cell as well as outside the cell (isotonic). Keeping the concentration the same on both sides of the cell membrane allows water to move out at a slow enough rate to prevent cell shrinkage, keeps the p. H and salt concentration at the right levels, and prevents damaging ice crystal formation (i. e. supercooling).

Different Kinds of Cryoprotectants • • Glycerol* Dimethylsulfoxide (DMSO) Polyvinylpyrrolidone (PVP) Proline Dextran Ethylene Glycol Propylene Glycol Sugars (Trehalose) or Skim Milk Different protocols use different cryoprotectants. Use what works for you.

Things You Need For Cryopreservation 1. Dewers and Liquid N 2 Something to store cells/tissues/cultures at or below -1960 C 2. Cryoprotectant Protect the cells/tissues/cultures from ice crystals and osmotic stress 3. Cryotubes/Cryovials Containers to hold the individual frozen cultures 4. Freeze containers, machines A way to rapidly bring cultures down in temperature but at the right speed 5. Water bucket, thermometer, and water at the right temperature A way to thaw them without damaging them

Where do you get the stuff you need? • All the major science supply firms like Fisher, VWR, and Cole-Parmer stock tubes, canes, gloves, freezers, etc. • Check local suppliers for LN 2

Fig. 1 Preparation of Chytrid Cultures For Freezing • Experiment! Some chytrids, mainly the monocentrics, can be frozen straight from thalli grown on agar media but…. . • A lot of chytrids, especially the polycentrics, have to be grown in liquid culture. We use Erlenmeyer flasks sealed with a cotton plug covered by aluminum foil as shown in Fig. 1

• For the flask cultures we use a Gyrotory Shaker at a constant low speed • We also use a variety of methods to freeze our cultures as there is no one universal method that works for all chytrids (See slides 23 and 24) • Again to get good results it will take some experimentation to find the right freezing protocol Fig. 2 Gyrotory Shaker with culture flasks

• One of the culture methods we use involves cutting off the cotton heads of Q-tips (Barr and Babcock, 1994) • The Q-tip increases the amount of surface area for colonization, increasing the amount of inoculum and the chances for recovery

Canes and Plastic Covers The cryotubes snap into the metal cane holding them in place. In addition, a plastic cover is used to keep the tubes from coming loose and rolling around in the canister inside the dewer.
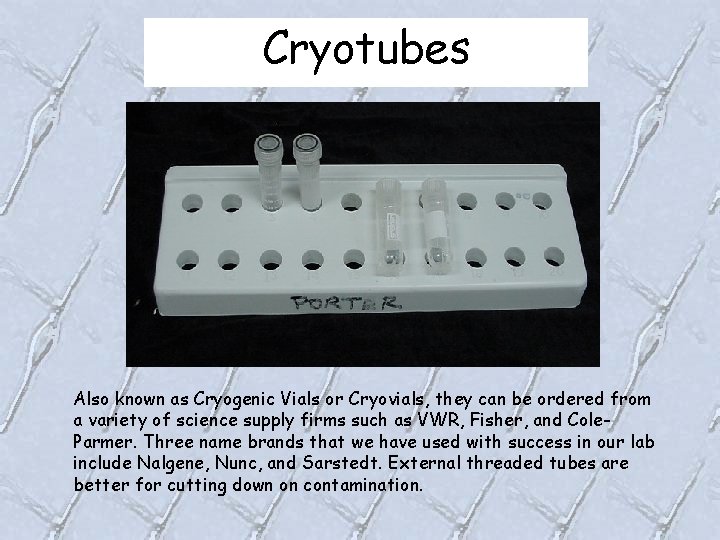
Cryotubes Also known as Cryogenic Vials or Cryovials, they can be ordered from a variety of science supply firms such as VWR, Fisher, and Cole. Parmer. Three name brands that we have used with success in our lab include Nalgene, Nunc, and Sarstedt. External threaded tubes are better for cutting down on contamination.

Nalgene Vial Freezing Container This is a cheap alternative to the large and expensive freezing machines as it only requires Isopropyl alcohol and a mechanical freezer (We use a – 800 C freezer) to freeze samples at a rate of 10 C/min and it can hold up to 18 1. 2 -2. 0 ml cryotubes

Our Dewar is a Taylor-Wharton storage vessel. It holds 6 canisters immersed in LN 2.

An inside view of the Dewer. Each canister has a different color-coded rubber-tipped handle.

Individual Canister Plastic Covered Canes carrying cryotubes

We use a simple method to thaw frozen samples for retrieval Metal bucket Thermometer

Thawing method • Run hot and cold water in a container (we use a metal bucket but anything will do) until the temperature reaches 350 C • Quickly retrieve your samples from the Dewer and drop them into the warm water *Warning! Wear safety glasses or turn away when dropping the tubes into the water as they can explode due to the large temperature change • Wearing gloves, hold the tube in the warm water until the contents of the tube become liquid but be careful! Don’t let the tube get too warm! • Either pour the contents into a liquid culture flask or place the inoculum (agar block, q-tip) onto one agar plate and transfer the liquid cryoprotectant to a second plate • If using plates, make sure to add 1 -3 ml of sterile DS or dd. H 2 O and rinsing the inoculum in a small amount of sterile dd. H 2 O before plating • Both of these measures will help ensure growth of the culture by diluting the cryoprotectant and providing the necessary moisture for growth, development, and reproduction

It really works!

Safety Tips • Always wear gloves to keep contamination down and prevent LN 2 injuries • Be careful with the Glycerol, Isopropyl alcohol, and LN 2 – all three are considered to be hazardous chemicals!

The following slides are a small experiment I did on freezing some of my Nowakowskiella clade chytrid cultures. If you have any questions or suggestions, feel free to contact me at: emozley@plantbio. uga. edu

Cryopreservation of members of the Nowakowskiella Clade • Methods used: 1. Skim Milk Protocol 2. Barr and Babcock (1994) Q-tip Method • Taxa used: • Monocentric • G-75 (Chytriomyces hyalinus/Chytridium conferve) • Endochytrium #75 JEL • Nephrochytrium #36 JEL • Polycentric • Cladochytrium replicatum #14 s Carmen Goal: To determine the best method for freezing Nowakowskiella clade cultures

Experimental Design • Cultures were provided by Dr. Joyce E. Longcore, University of Maine, Orono, ME. • Three monocentric cultures and one polycentric culture were frozen using two different methods to assess which method would be the best for freezing members of the Nowakowskiella clade for long term storage. • Cultures were grown up on plates for the Skim Milk method while they were grown in broth media for the Barr and Babcock method. • Cultures were frozen at 3, 4, and 5 days for the Skim Milk method as it is recommended to freeze younger cultures. • Growth took longer in the broth media (Generally one to two weeks) with the Q-tips for the Barr and Babcock method.

Skim Milk Method 1. Make 200 ml of cryoprotectant Final Concentrations: Glycerol 10% Skim Milk 8. 5% 2. Pipet 1. 5 ml of cryoprotectant into a cryotube. 3. Add a small cube of agar with inoculum to the cryotube. 4. Place tube in the Nalgene container. 5. Place the container in a -800 C freezer for at least three days 6. After three days, place the cryotube into a LN 2 Dewar for long term storage. 7. For Recovery: Heat water bath to 430 C, quickly remove tubes from Dewar, place carefully into water bath and remove once the contents are thawed. Dump contents out onto an agar plate, move agar block to a separate plate. Flood both plates with ~ 1 ml of dd. H 2 O/DS/Seawater*. Watch for 3 -4 weeks. USE ANTIBIOTICS IN MEDIA! We routinely use Penicillin and Streptomycin at a concentration of 0. 25 g/L.

Barr and Babcock Q-tip Method • Place 10 -20 Q-tip heads into a 250 ml Erlenmeyer Flask • Add 100 ml of YPD broth • Autoclave • Inoculate and incubate at appropriate temperature for 3 -5 days • Pipet some 10%Glycerol into a 2 ml plastic screw cap cryotube • Place one Q-tip head into each tube • Freeze by storing in the vapor phase in a LN 2 refridgerator* • For Recovery: Thaw briefly in a 350 C water bath and place Q-tip into 100 ml of suitable broth • Pre-Chill broth for species with a lower than ambient optimum temperature • Check microscopically within 36 -48 hrs Barr, D. J. S. and Babcock, C. E. Culture Collection Information: Cryopreservation of Unicellular Zoosporic Fungi. U. S. Federation of Culture Collections Newsletter. 24(2): 6. 1994. *Storage in the vapor phase can lead to an increased risk of contamination. The way we store cultures, in canisters immersed in LN 2, is a better alternative.

Results of Skim Milk Method on Nowakowskiella Clade cultures + culture grew - culture did not grow na could not grow up enough to freeze nt tube still frozen • G 75, 36, and 75 were all successfully revived from frozen cultures • 14 s did not survive the freezing process
Results of Barr and Babcock Q-Tip Method on Nowakowskiella Clade cultures • The first trial repeated the results of the Skim Milk method: Both G 75 and 36 survived the freezing process while 14 s did not. Unfortunately, 75 did not grow at all in the broth. • Surprisingly enough, in the second trial 14 s was successfully revived from the frozen samples.

In Conclusion • • Monocentrics generally freeze well irrespective of method used (i. e. G 75, 36, 75) Polycentrics, on the other hand, seem to require the Barr and Babcock Q-tip method (14 s) Unfortunately, some cultures may not freeze well no matter what you do Chytrid cryoparameter work (parameters required for freezing) needs to be done in order to successfully freeze all isolates

Additional Resources • Biological Effects of Freezing and Supercooling, Smith 1961 • Cryobiology, Meryman 1966 • Current Trends in Cryobiology, Smith 1970 • The Frozen Cell, Wolstenholme and O’Connor 1970 • Low Temperature Preservation in Medicine and Biology, Ashwood-Smith and Farrant 1980 • ATCC Preservation Methods: Freezing and Freeze-Drying, Simione and Brown 1991 • Advances in Low Temperature Biology, Steponkus 1993 • Cryopreservation and Freeze-Drying Protocols, Day and Mc. Lellan 1995 • Reproductive Tissue Banking: Scientific Principles Karow and Critser 1997 • Asymptote Cool Guide to Cryopreservation - An Online Resource

More Additional Resources…. . ZFTR (Zoosporic Fungi in Teaching and Research, Eds. Jaworski and Fuller, 1987) Canadian Collection of Fungal Cultures - D. J. S. Barr http: //sis. agr. gc. ca/brd/ccc/ Rob Specker, University of Georgia, Algal and Fungal Culture Curator, (706)542 -1783, specker@plantbio. uga. edu - Lots of experience in freezing chytrids, oomycetes, etc. . . Ryan, M. J. , Smith, D. , and Jefferies, P. A decision based key to determine the most appropriate protocol for the preservation of fungi. World Journal of Microbiology and Biotechnology. 16(2): 183 -186. 2000.
- Slides: 30