A STUDY OF REACTION RATES CHEMICAL KINETICS WHAT


![HOW DO YOU MEASURE REACTION RATES? What happens to the [reactants] over time? What HOW DO YOU MEASURE REACTION RATES? What happens to the [reactants] over time? What](https://slidetodoc.com/presentation_image_h2/2728b473d1407080038858122726577a/image-6.jpg)







- Slides: 19

A STUDY OF REACTION RATES CHEMICAL KINETICS

WHAT IS KINETICS? The study of the rate at which a chemical reaction takes place. It provides a clue to how a chemical reaction takes place – this is the mechanism or pathway of the reaction.

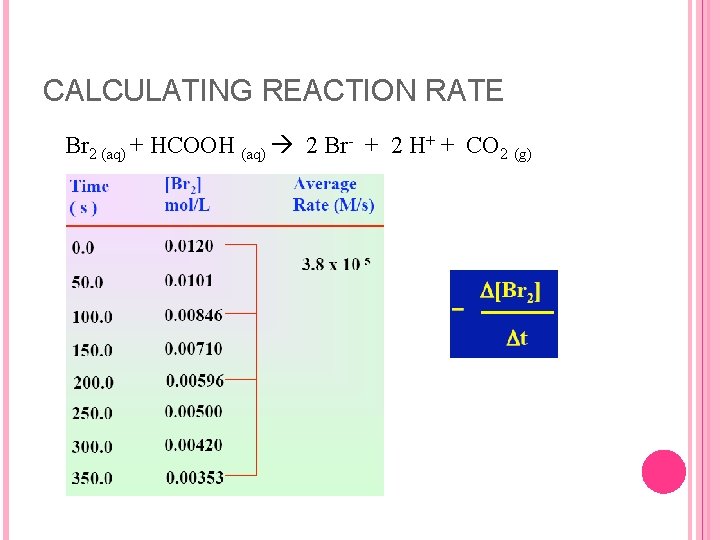
CALCULATING REACTION RATE Br 2 (aq) + HCOOH (aq) 2 Br- + 2 H+ + CO 2 (g)

REACTANT CONCENTRATION VS TIME

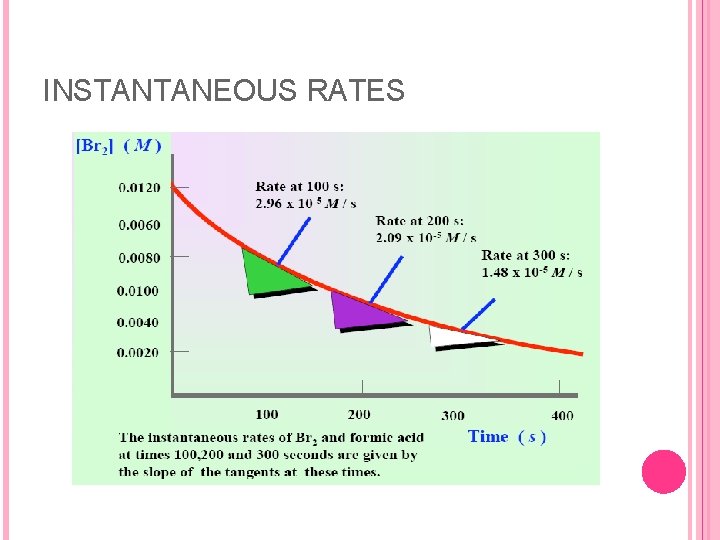
INSTANTANEOUS RATES
![HOW DO YOU MEASURE REACTION RATES What happens to the reactants over time What HOW DO YOU MEASURE REACTION RATES? What happens to the [reactants] over time? What](https://slidetodoc.com/presentation_image_h2/2728b473d1407080038858122726577a/image-6.jpg)
HOW DO YOU MEASURE REACTION RATES? What happens to the [reactants] over time? What happens to the [products] over time? H 2 + I 2 2 HI

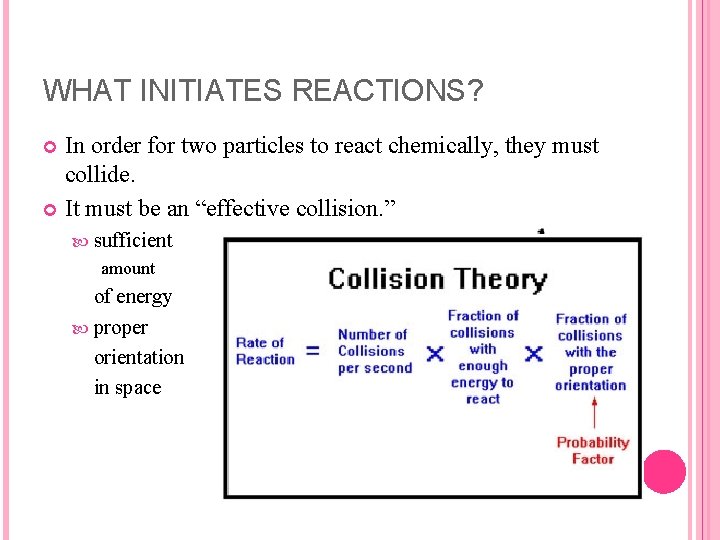
WHAT INITIATES REACTIONS? In order for two particles to react chemically, they must collide. It must be an “effective collision. ” sufficient amount of energy proper orientation in space

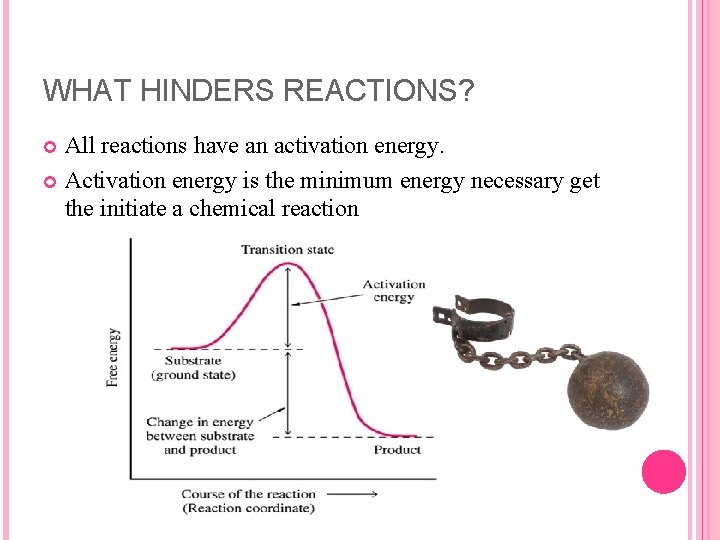
WHAT HINDERS REACTIONS? All reactions have an activation energy. Activation energy is the minimum energy necessary get the initiate a chemical reaction

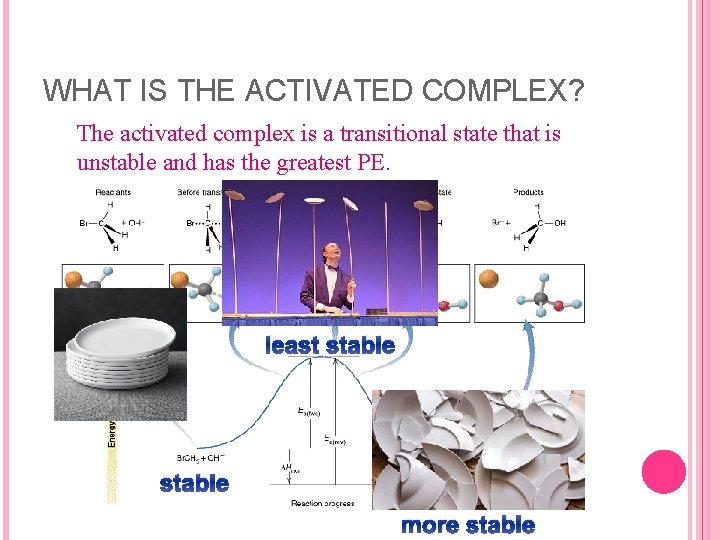
WHAT IS THE ACTIVATED COMPLEX? The activated complex is a transitional state that is unstable and has the greatest PE. least stable more stable

WHY DOES THE PE INCREASE? Isomerization of isonitrile: CH 3 NC BTW, why is CH 3 CN more stable than CH 3 NC? Number of bonds made by C atoms vs number of bonds made by N atoms? Bond Energy C−N vs C−C? 293 vs 348 k. J CH 3 NC CH 3 CN

WHAT FACTORS AFFECT RATES? Surface Area/ Contact Area Concentration Catalyst Temperature Nature of reaction

HOW DOES SURFACE AREA AFFECT RATE? More surface area exposes more reactants to collisions. Gases react quickly. Solids react slowly unless ground into very fine powder. Fireworks take advantage of this concept.

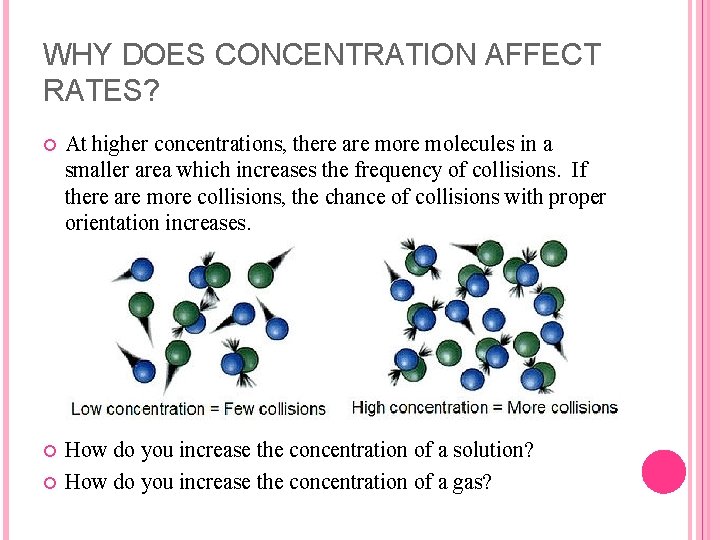
WHY DOES CONCENTRATION AFFECT RATES? At higher concentrations, there are molecules in a smaller area which increases the frequency of collisions. If there are more collisions, the chance of collisions with proper orientation increases. How do you increase the concentration of a solution? How do you increase the concentration of a gas?

HOW DOES A CATALYST AFFECT RATE? A catalyst helps to orient the reactants for a proper collision. It provides a surface for the reactants to react. A catalyst does not get consumed in the reaction.

HOW DOES LOWERING EA AFFECT RATE?

WHAT DO CATALYTIC CONVERTERS DO? It reduces the harmful emissions (CO, Cx. Hy, NOx) into less harmful emissions (N 2, H 2 O, CO 2).

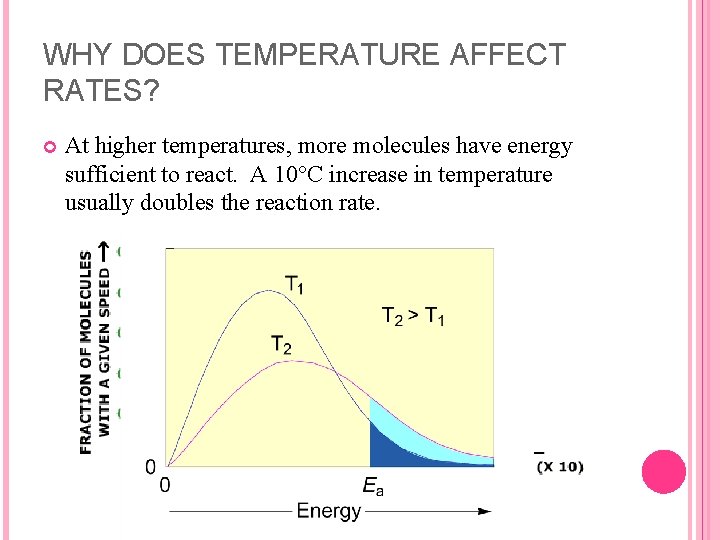
WHY DOES TEMPERATURE AFFECT RATES? At higher temperatures, more molecules have energy sufficient to react. A 10°C increase in temperature usually doubles the reaction rate.

HOW DO TYPE OF REACTANTS AFFECT RATE? Ions react faster than molecule because of the attraction of opposite ions. Molecules with more bonds take longer to react since more bonds must be broken. Molecules with stronger bonds take more energy to break and therefore, take longer to react

WHAT FACTORS AFFECT RATES? Surface Area/ Contact Area Put yourself out there! (Increase Frequency - More Opportunities for Collisions) Concentration Go where the potential partners are! (Increase Frequency) Catalyst Get someone to make the introductions! (Effective Collisions – Collisions w/ Proper Orientations) Temperature Bring your “A” game or improve your game! (Increase Frequency) Nature of reactants (Increase Frequency) Be true to yourself!