A solution is a homogeneous mixture that is


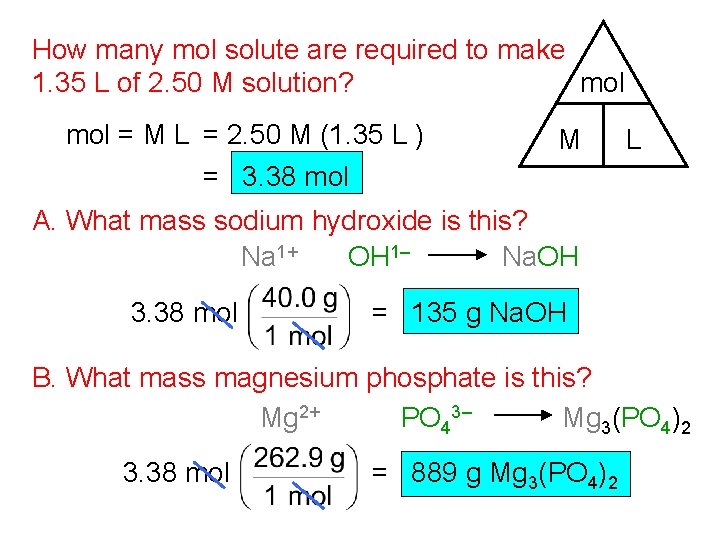








- Slides: 33


A solution is a homogeneous mixture that is the same all the way throughout. Water and Soda is an example of a homogeneous mixture. Homogeneous mixtures do not settle out if left to sit undisturbed, whereas a heterogeneous mixture would. In a heterogeneous mixture you can see all of the parts of the solution. Orange Juice with pulp is an example of a heterogeneous mixture. A solution has two components: the solute and the solvent. The solute is the substance in lesser amount. It is the substance that is being dissolved. The solvent is the substance in greater amount. It is the substance that is doing the dissolving.

Concentration…a measure of solute-to-solvent ratio concentrated dilute “lots of solute” “not much solvent” “watery” Add water to dilute a solution; boil water off to concentrate it.

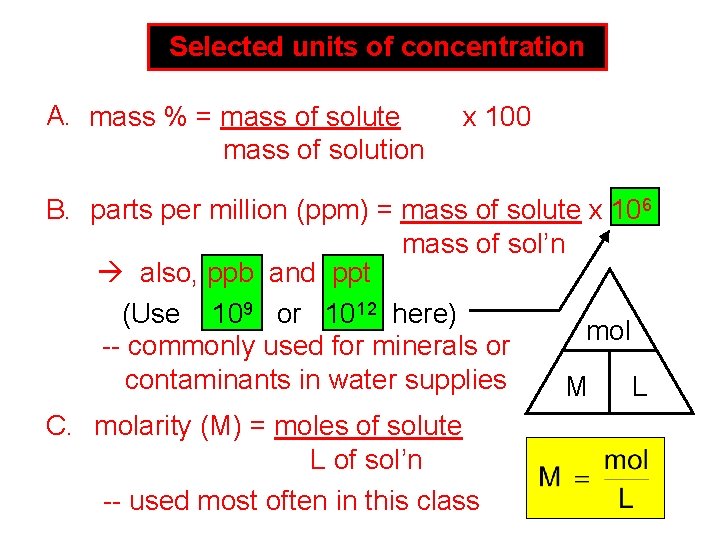
Selected units of concentration A. mass % = mass of solute mass of solution x 100 B. parts per million (ppm) = mass of solute x 106 mass of sol’n also, ppb and ppt (Use 109 or 1012 here) mol -- commonly used for minerals or contaminants in water supplies M L C. molarity (M) = moles of solute L of sol’n -- used most often in this class

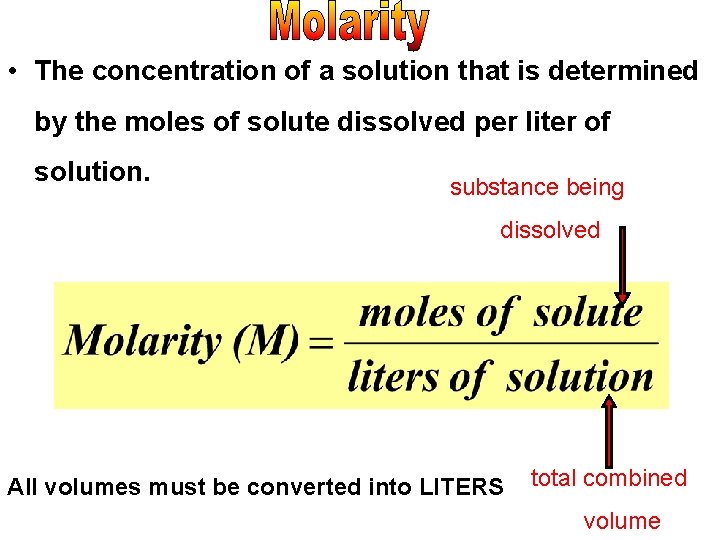
• The concentration of a solution that is determined by the moles of solute dissolved per liter of solution. substance being dissolved All volumes must be converted into LITERS total combined volume

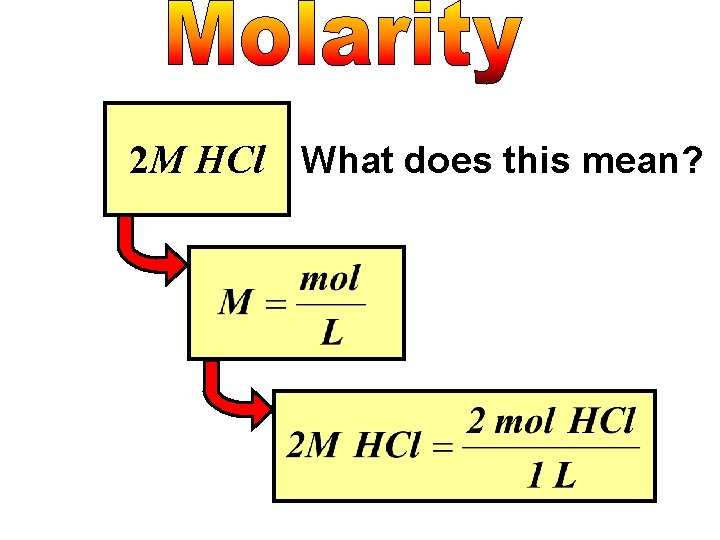
2 M HCl What does this mean?

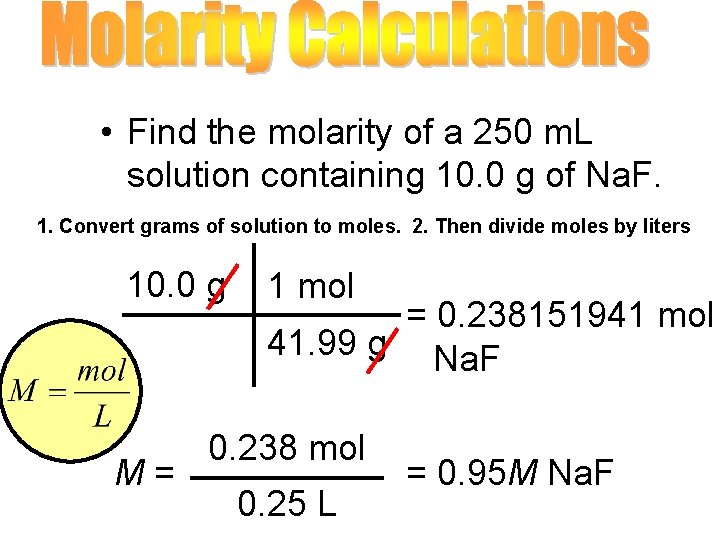
• Find the molarity of a 250 m. L solution containing 10. 0 g of Na. F. 1. Convert grams of solution to moles. 2. Then divide moles by liters 10. 0 g M= 1 mol = 0. 238151941 mol 41. 99 g Na. F 0. 238 mol 0. 25 L = 0. 95 M Na. F

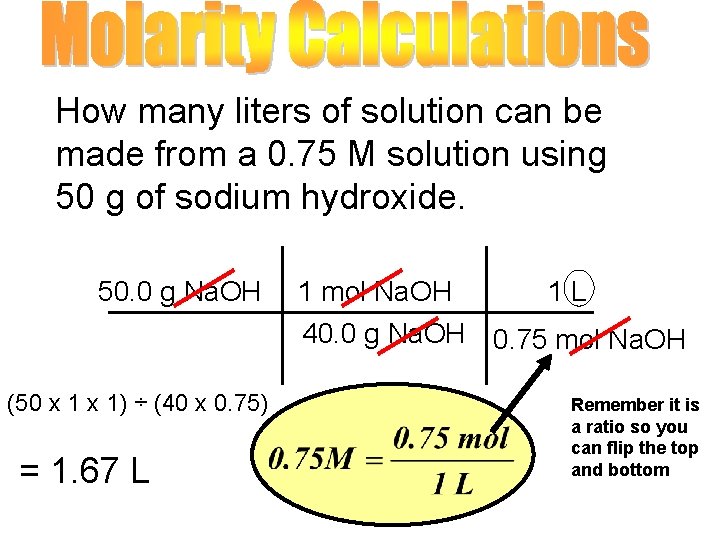
How many liters of solution can be made from a 0. 75 M solution using 50 g of sodium hydroxide. 50. 0 g Na. OH (50 x 1) ÷ (40 x 0. 75) = 1. 67 L 1 mol Na. OH 1 L 40. 0 g Na. OH 0. 75 mol Na. OH Remember it is a ratio so you can flip the top and bottom

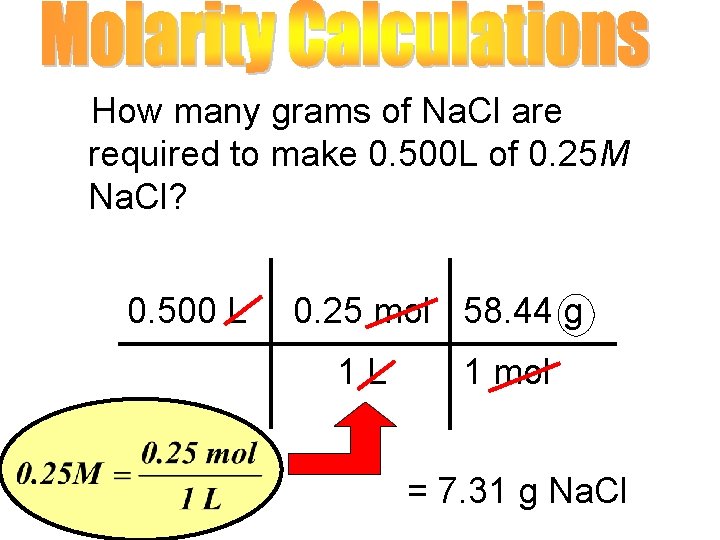
How many grams of Na. Cl are required to make 0. 500 L of 0. 25 M Na. Cl? 0. 500 L 0. 25 mol 58. 44 g 1 L 1 mol = 7. 31 g Na. Cl
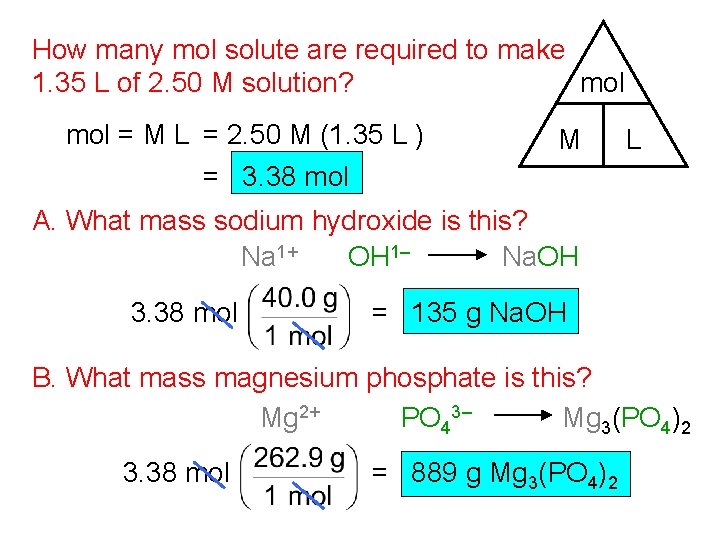
How many mol solute are required to make mol 1. 35 L of 2. 50 M solution? mol = M L = 2. 50 M (1. 35 L ) = 3. 38 mol M L A. What mass sodium hydroxide is this? Na 1+ OH 1– Na. OH 3. 38 mol = 135 g Na. OH B. What mass magnesium phosphate is this? Mg 2+ PO 43– Mg 3(PO 4)2 3. 38 mol = 889 g Mg 3(PO 4)2

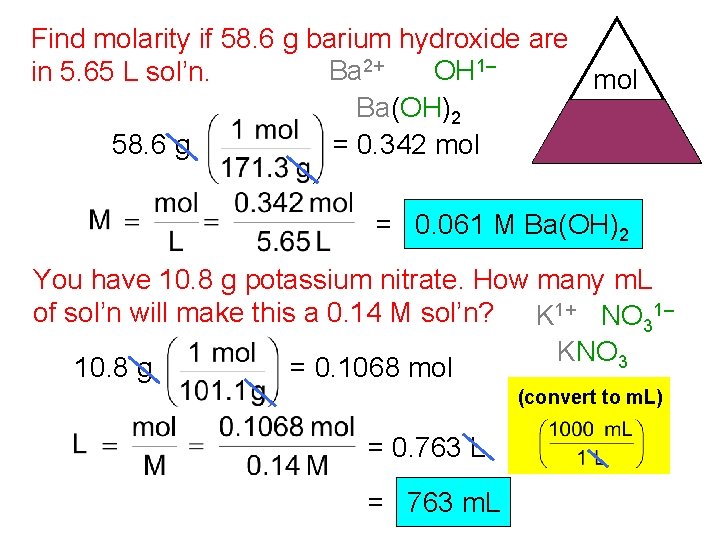
Find molarity if 58. 6 g barium hydroxide are Ba 2+ OH 1– in 5. 65 L sol’n. mol Ba(OH)2 M L 58. 6 g = 0. 342 mol = 0. 061 M Ba(OH)2 You have 10. 8 g potassium nitrate. How many m. L of sol’n will make this a 0. 14 M sol’n? K 1+ NO 31– KNO 3 10. 8 g = 0. 1068 mol (convert to m. L) = 0. 763 L = 763 m. L


How do reactions occur? Collision Theory In order for a chemical reaction to take place, the reactants must collide. The collision transfers kinetic energy needed to break the necessary bonds so that new bonds can be formed.

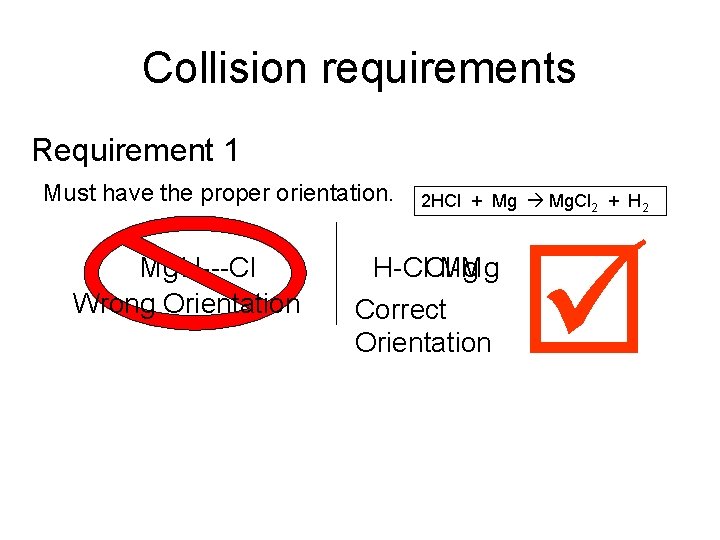
Collision requirements Requirement 1 Must have the proper orientation. Mg H---Cl Wrong Orientation 2 HCl + Mg Mg. Cl 2 + H 2 H-Cl. Cl-Mg H Mg Correct Orientation

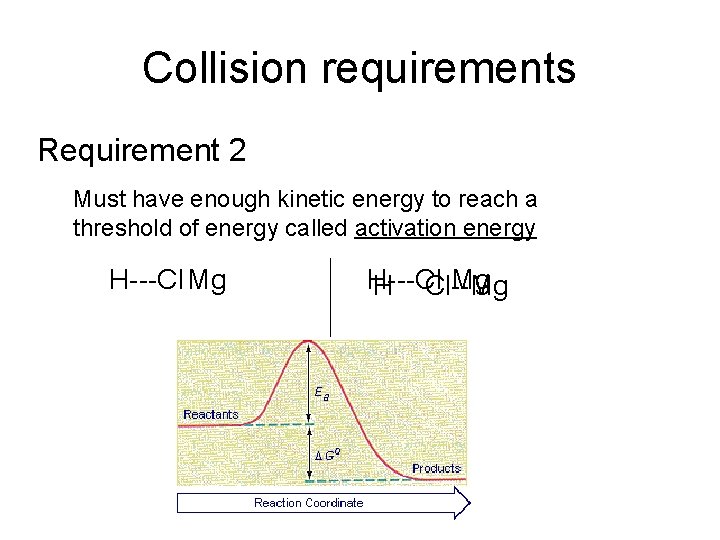
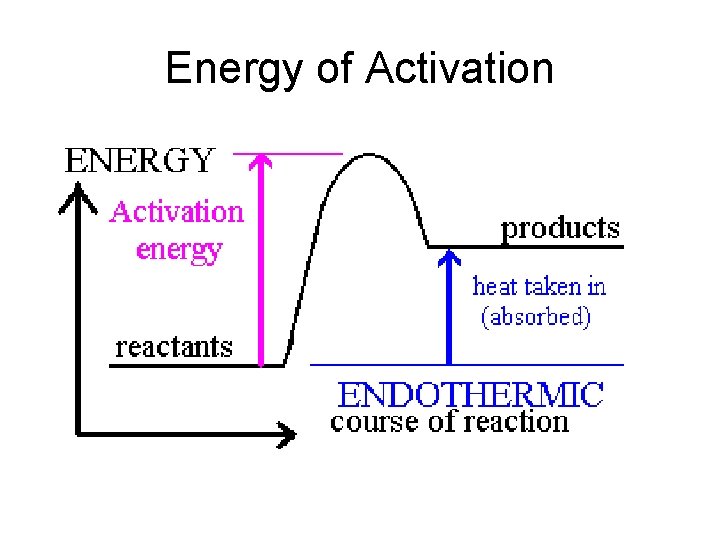
Collision requirements Requirement 2 Must have enough kinetic energy to reach a threshold of energy called activation energy H---Cl Mg H Cl--Mg

Energy of Activation

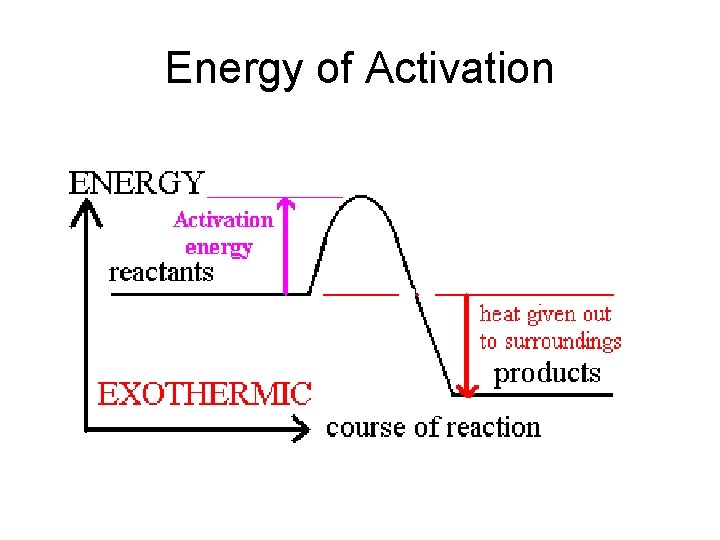
Energy of Activation

Factors Affecting Reaction Rate • Surface Area –high SA = fast rxn rate –more opportunities for collisions –Increase surface area by… • using smaller particles (crush the substance into pieces) • use a liquid rather than a solid • dissolving in water

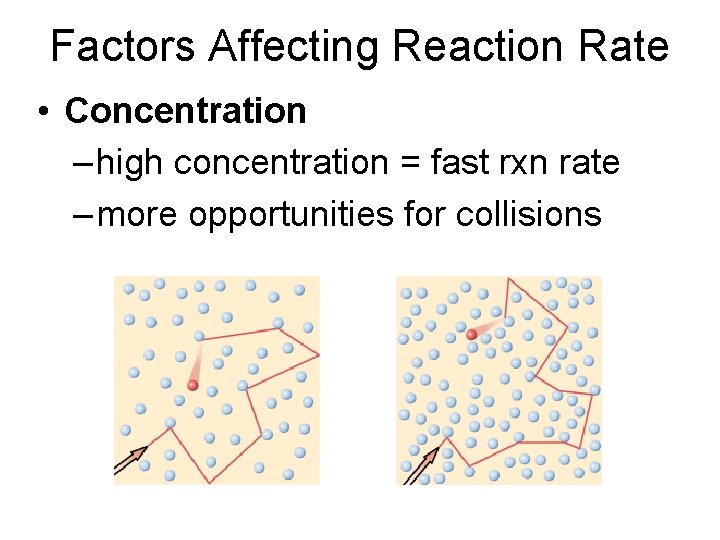
Factors Affecting Reaction Rate • Concentration – high concentration = fast rxn rate – more opportunities for collisions

Factors Affecting Reaction Rate • Temperature –high temp = fast reaction rate –high Kinetic Energy • fast-moving particles • more collisions • more likely to reach activation energy

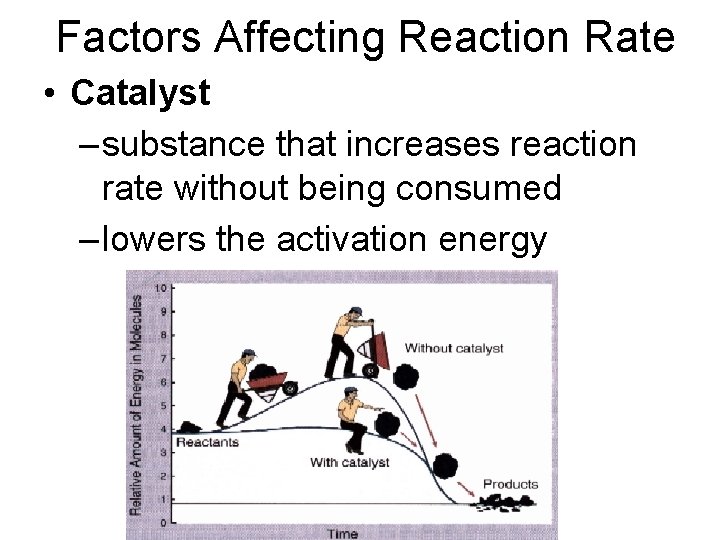
Factors Affecting Reaction Rate • Catalyst – substance that increases reaction rate without being consumed – lowers the activation energy

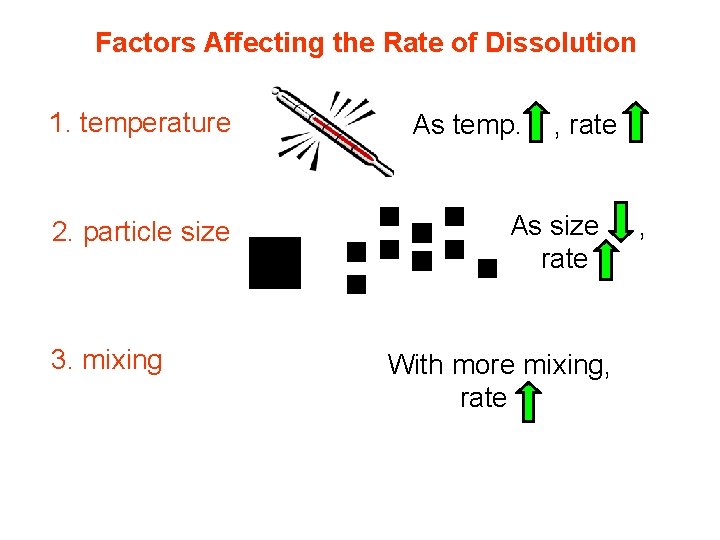
Factors Affecting the Rate of Dissolution 1. temperature 2. particle size 3. mixing As temp. , rate As size rate With more mixing, rate ,


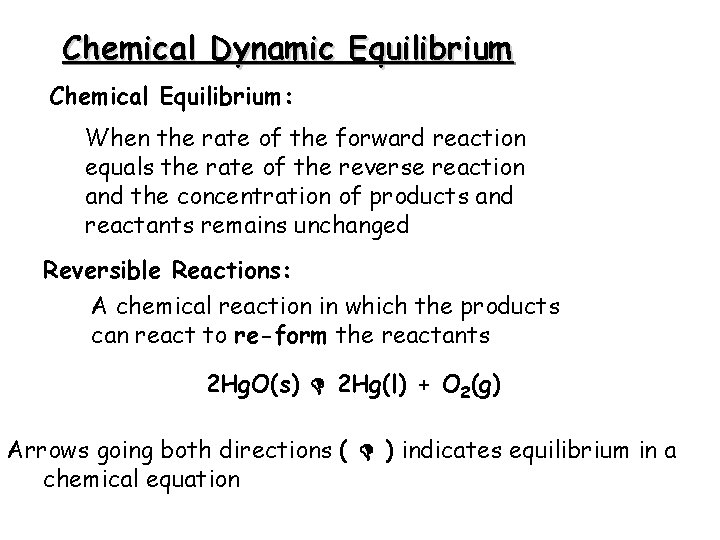
Chemical Dynamic Equilibrium Chemical Equilibrium: When the rate of the forward reaction equals the rate of the reverse reaction and the concentration of products and reactants remains unchanged Reversible Reactions: A chemical reaction in which the products can react to re-form the reactants 2 Hg. O(s) 2 Hg(l) + O 2(g) Arrows going both directions ( ) indicates equilibrium in a chemical equation

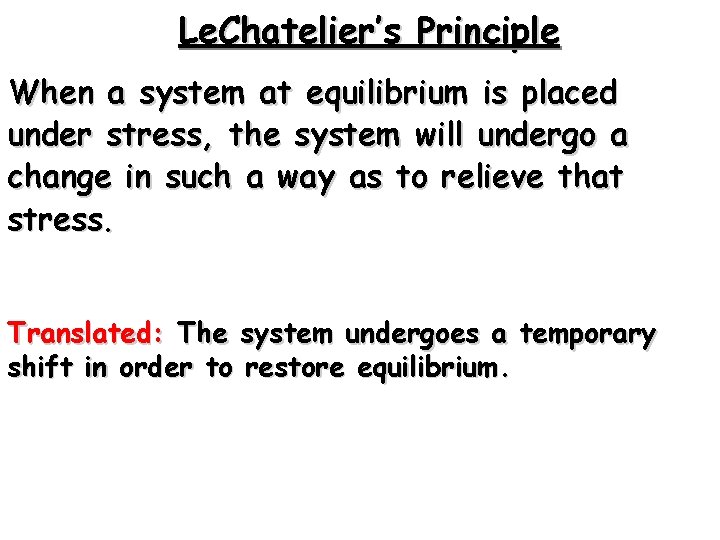
Le. Chatelier’s Principle When a system at equilibrium is placed under stress, the system will undergo a change in such a way as to relieve that stress. Translated: The system undergoes a temporary shift in order to restore equilibrium.

1) Effect of change of concentration By increasing or decreasing the concentration of one of the reactants or products, the reaction can be manipulated in the desired direction. If a reactant is added the reaction will shift RIGHT If a reactant is removed the reaction will shift LEFT If a product is added the reaction will shift LEFT If a product is removed the reaction will shift RIGHT N 2 + 3 H 2 2 NH 3 If more N 2 is added to the reaction which way will it shift? If H 2 is removed from the reaction which way will it shift? RIGHT LEFT

3) Effect of change in temperature If the temperature increases, the reaction will shift to favor the endothermic (side without energy) If the temperature decreases, the reaction will shift to favor the exothermic (side with energy) N 2 + 3 H 2 2 NH 3 + energy If the temperature in the reaction increases which way will it shift? LEFT 3 O 2 2 O 3 + energy If the temperature in the reaction decreases which way will it shift? RIGHT

2) Effect of change of pressure If the pressure increases, the reaction will shift to favor the side with the least number of molecules If the pressure decreases, the reaction will shift to favor the side with the most number of molecules If the number of molecules on both sides of the reaction is equal, there is no change. 2 SO 2 + O 2 2 SO 3 If the pressure increases in the reaction which way will it shift? RIGHT 2 H 2 O 2 H 2 + O 2 If the pressure decreases in the reaction which way will it shift? RIGHT

4) Effect of Catalyst The presence of a catalyst in a reversible reaction does not affect the state of equilibrium but only causes the reaction system to attain equilibrium in a shorter period of time.

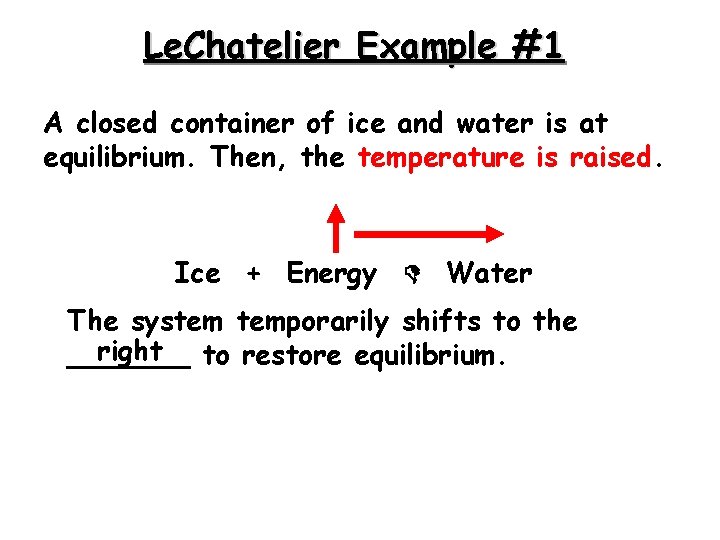
Le. Chatelier Example #1 A closed container of ice and water is at equilibrium. Then, the temperature is raised. Ice + Energy Water The system temporarily shifts to the right to restore equilibrium. _______

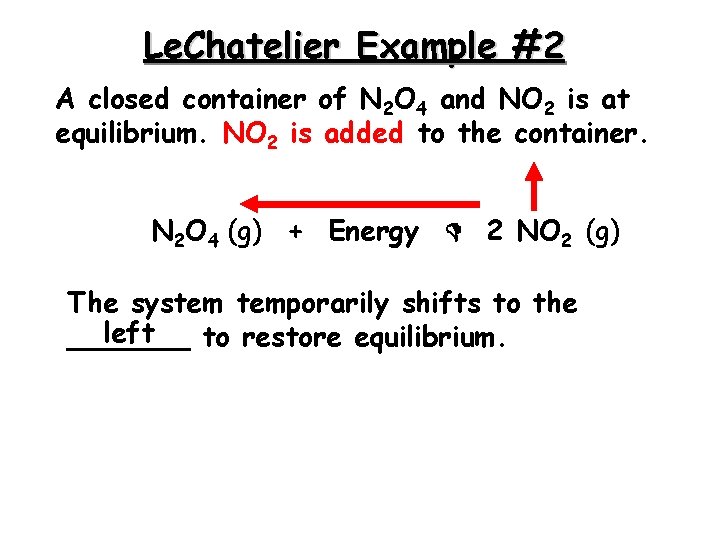
Le. Chatelier Example #2 A closed container of N 2 O 4 and NO 2 is at equilibrium. NO 2 is added to the container. N 2 O 4 (g) + Energy 2 NO 2 (g) The system temporarily shifts to the left _______ to restore equilibrium.

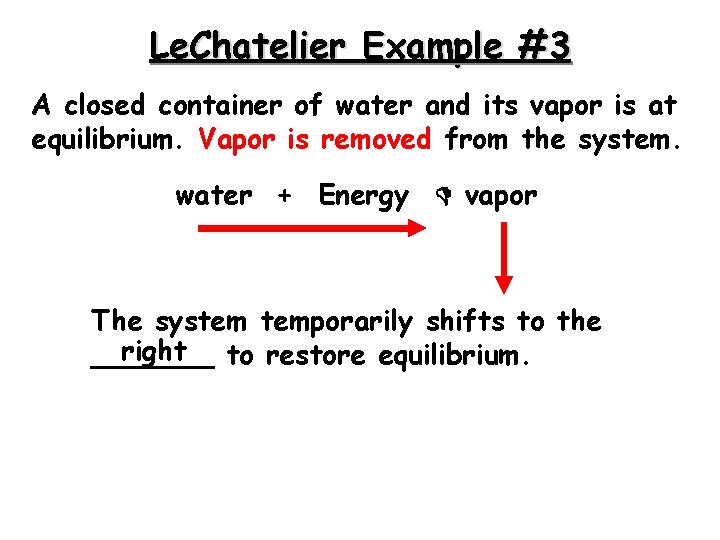
Le. Chatelier Example #3 A closed container of water and its vapor is at equilibrium. Vapor is removed from the system. water + Energy vapor The system temporarily shifts to the right to restore equilibrium. _______

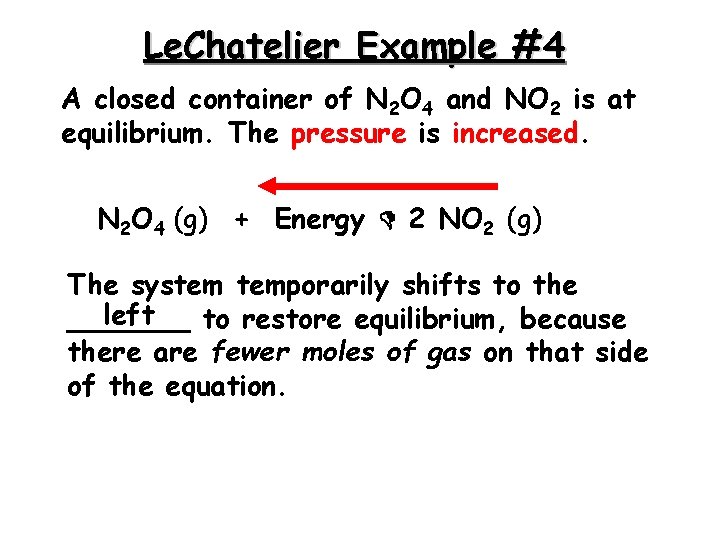
Le. Chatelier Example #4 A closed container of N 2 O 4 and NO 2 is at equilibrium. The pressure is increased. N 2 O 4 (g) + Energy 2 NO 2 (g) The system temporarily shifts to the left _______ to restore equilibrium, because there are fewer moles of gas on that side of the equation.