A Solution for Obstructive Sleep Apnea Closed Loop

“A Solution for Obstructive Sleep Apnea: Closed Loop Neuromodulation: ” Mark Christopherson Vice President Product Development, Inspire Medical Systems, Inc. NANS, December 6, 2012 • The Inspire Therapy is Investigational Use Only in the United States • Mark Christopherson is an employee of Inspire Medical Systems
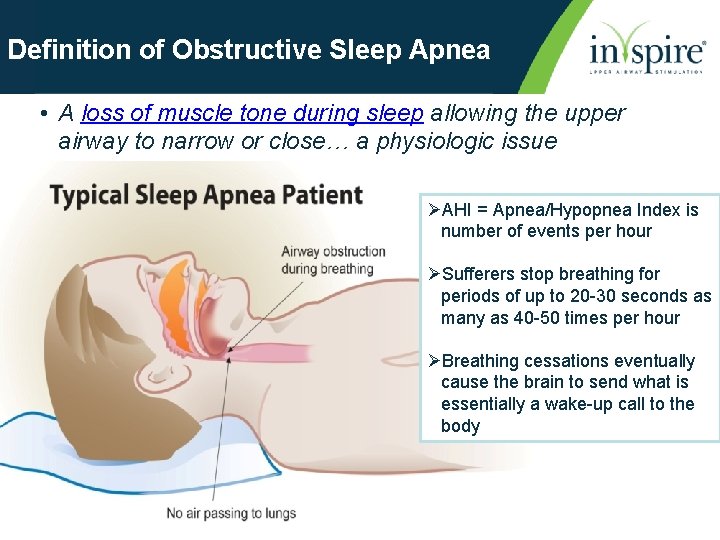
Definition of Obstructive Sleep Apnea • A loss of muscle tone during sleep allowing the upper airway to narrow or close… a physiologic issue ØAHI = Apnea/Hypopnea Index is number of events per hour ØSufferers stop breathing for periods of up to 20 -30 seconds as many as 40 -50 times per hour ØBreathing cessations eventually cause the brain to send what is essentially a wake-up call to the body

Health Consequences of OSA Many co-morbidities have been directly linked with Obstructive Sleep Apnea… Early Mortality High Blood Pressure Congestive Heart Failure Stroke Neuro-cognitive Problems Daytime Sleepiness Job Impairment Fatigue-related accidents
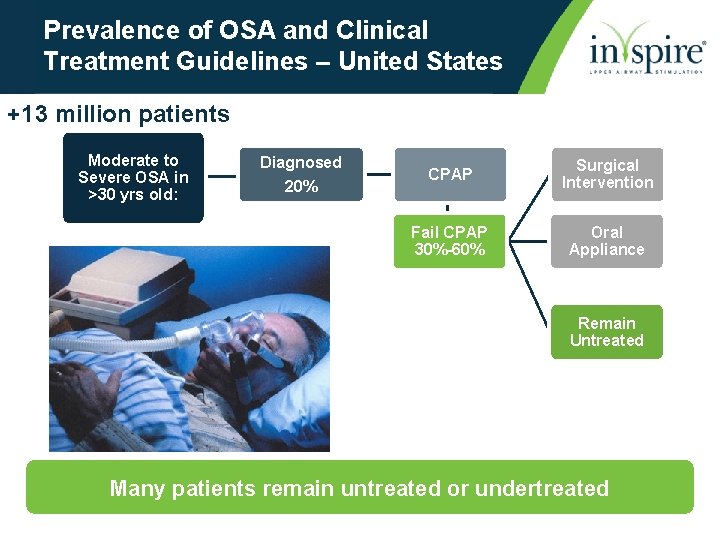
Prevalence of OSA and Clinical Treatment Guidelines – United States +13 million patients Moderate to Severe OSA in >30 yrs old: Diagnosed 20% CPAP Surgical Intervention Fail CPAP 30%-60% Oral Appliance Remain Untreated Many patients remain untreated or undertreated
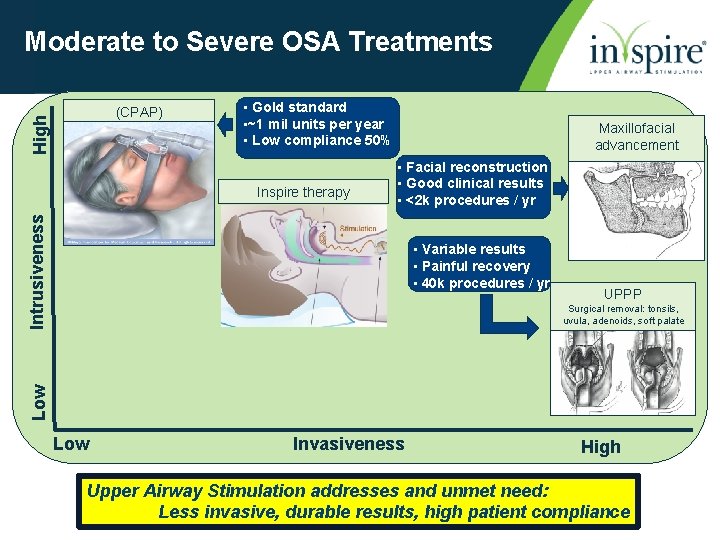
Moderate to Severe OSA Treatments High (CPAP) • Gold standard • ~1 mil units per year • Low compliance 50% • Facial reconstruction • Good clinical results • <2 k procedures / yr Intrusiveness Inspire therapy Maxillofacial advancement • Variable results • Painful recovery • 40 k procedures / yr UPPP Low Surgical removal: tonsils, uvula, adenoids, soft palate Low Invasiveness High Upper Airway Stimulation addresses and unmet need: Less invasive, durable results, high patient compliance
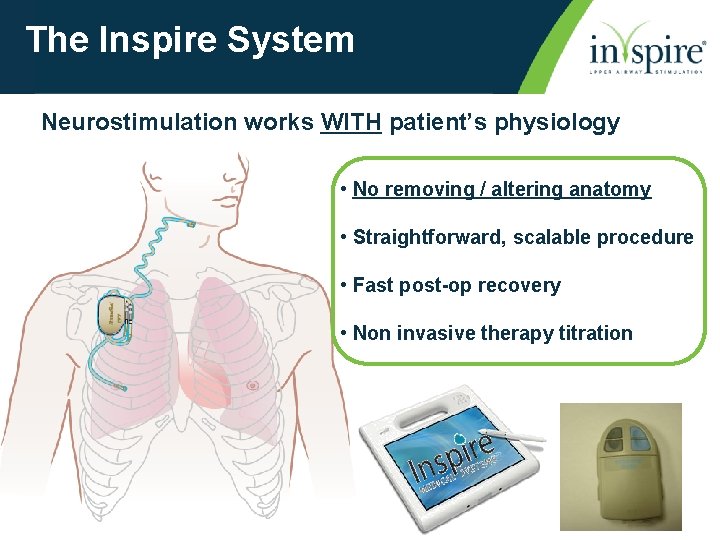
The Inspire System Neurostimulation works WITH patient’s physiology • No removing / altering anatomy • Straightforward, scalable procedure • Fast post-op recovery • Non invasive therapy titration
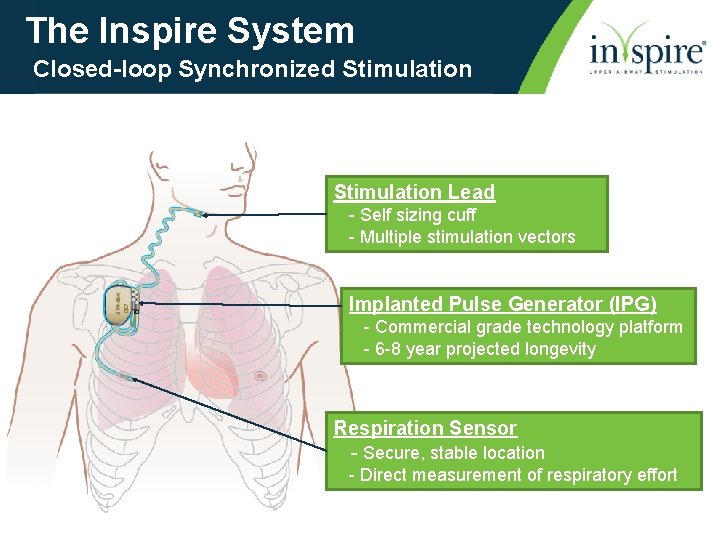
The Inspire System Closed-loop Synchronized Stimulation Lead - Self sizing cuff - Multiple stimulation vectors Implanted Pulse Generator (IPG) - Commercial grade technology platform - 6 -8 year projected longevity Respiration Sensor - Secure, stable location - Direct measurement of respiratory effort
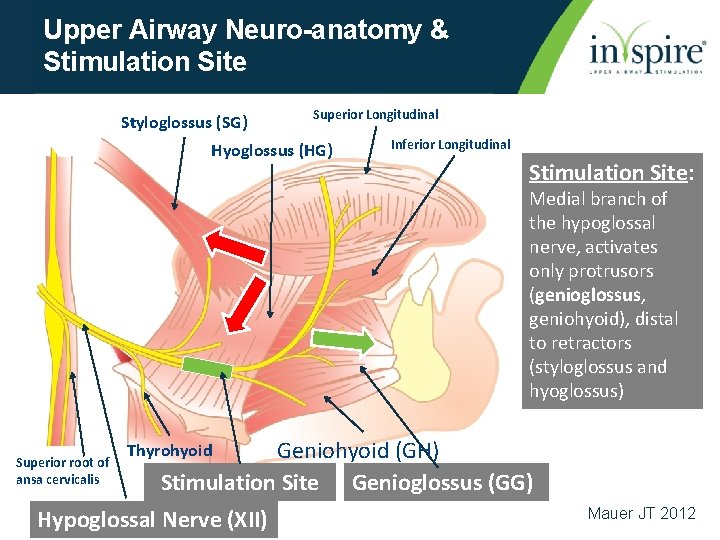
Upper Airway Neuro-anatomy & Stimulation Site Styloglossus (SG) Superior Longitudinal Hyoglossus (HG) Inferior Longitudinal Stimulation Site: Medial branch of the hypoglossal nerve, activates only protrusors (genioglossus, geniohyoid), distal to retractors (styloglossus and hyoglossus) Superior root of ansa cervicalis Geniohyoid (GH) Stimulation Site Genioglossus (GG) Thyrohyoid Hypoglossal Nerve (XII) Mauer JT 2012
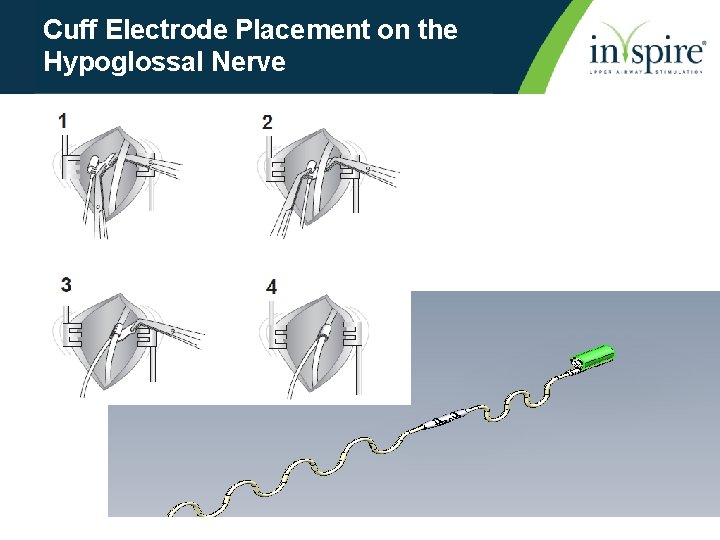
Cuff Electrode Placement on the Hypoglossal Nerve
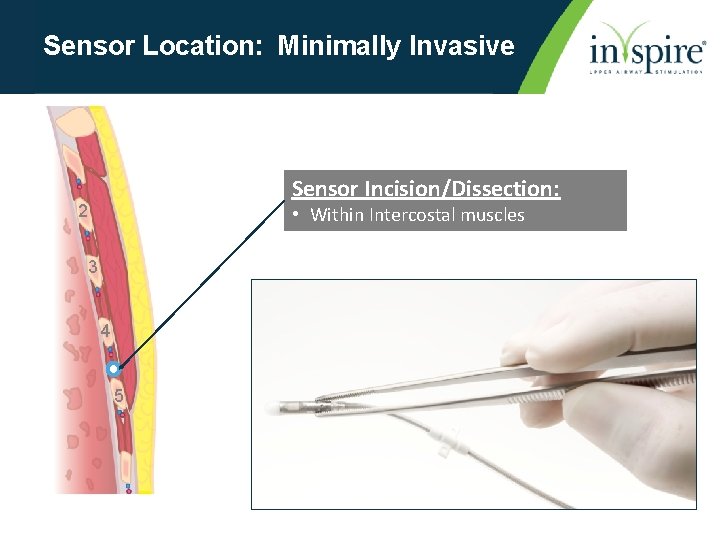
Sensor Location: Minimally Invasive Sensor Incision/Dissection: 2 • Within Intercostal muscles 3 4 5
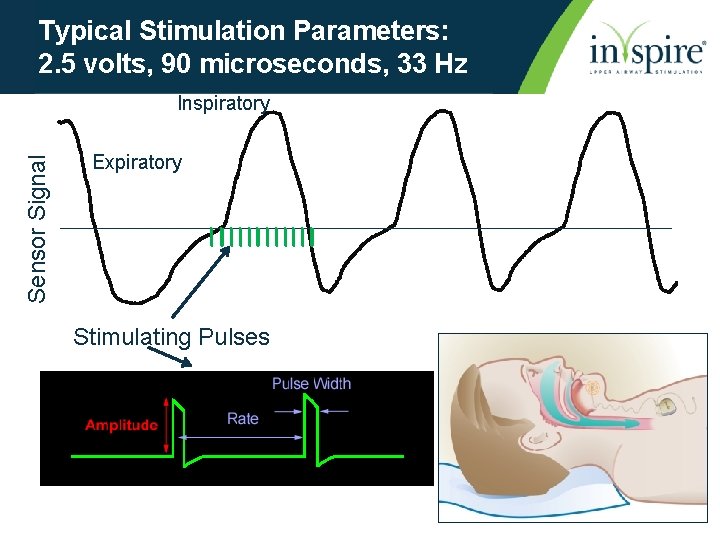
Typical Stimulation Parameters: 2. 5 volts, 90 microseconds, 33 Hz Sensor Signal Inspiratory Expiratory Stimulating Pulses
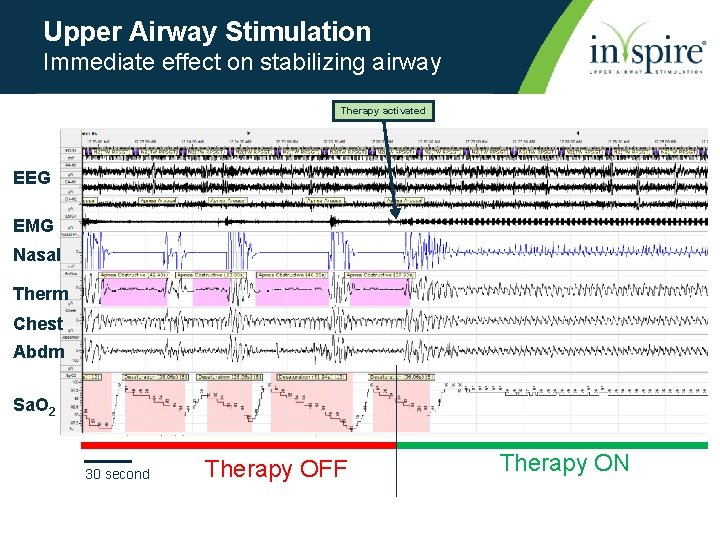
Upper Airway Stimulation Immediate effect on stabilizing airway Therapy activated EEG EMG Nasal Therm Chest Abdm Sa. O 2 30 second Therapy OFF Therapy ON
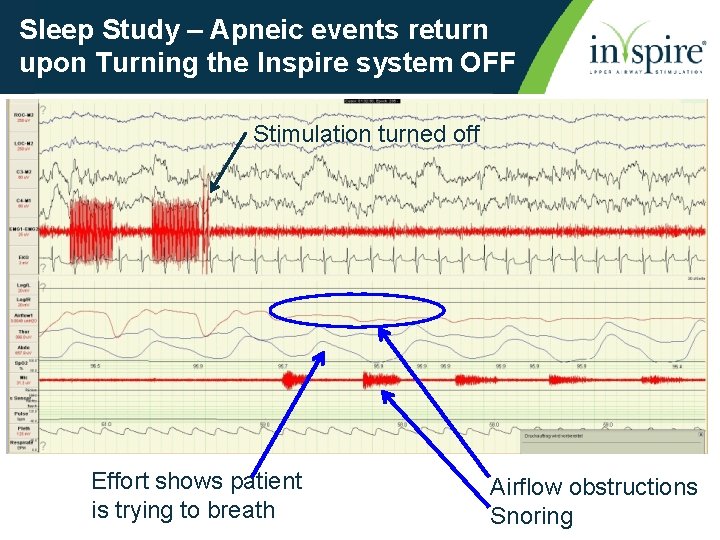
Sleep Study – Apneic events return upon Turning the Inspire system OFF Stimulation turned off Effort shows patient is trying to breath Airflow obstructions Snoring
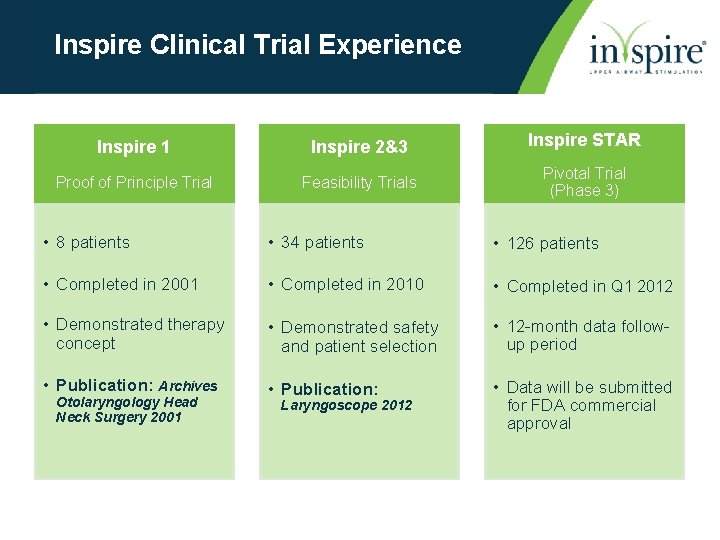
Inspire Clinical Trial Experience Inspire 1 Inspire 2&3 Inspire STAR Proof of Principle Trial Feasibility Trials Pivotal Trial (Phase 3) • 8 patients • 34 patients • 126 patients • Completed in 2001 • Completed in 2010 • Completed in Q 1 2012 • Demonstrated therapy concept • Demonstrated safety and patient selection • 12 -month data followup period • Publication: Archives • Publication: • Data will be submitted for FDA commercial approval Otolaryngology Head Neck Surgery 2001 Laryngoscope 2012
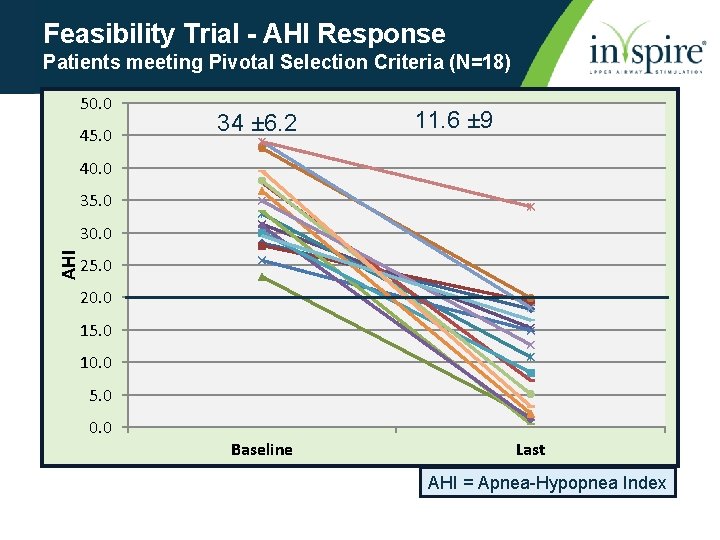
Feasibility Trial - AHI Response Patients meeting Pivotal Selection Criteria (N=18) 50. 0 45. 0 34 ± 6. 2 11. 6 ± 9 40. 0 35. 0 AHI 30. 0 25. 0 20. 0 15. 0 10. 0 5. 0 0. 0 Baseline Last AHI = Apnea-Hypopnea Index
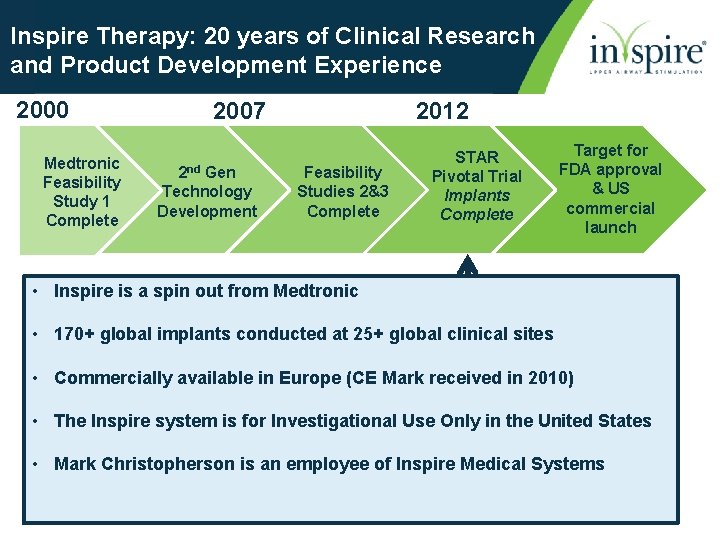
Inspire Therapy: 20 years of Clinical Research and Product Development Experience 2000 Medtronic Feasibility Study 1 Complete 2007 2 nd Gen Technology Development 2012 Feasibility Studies 2&3 Complete STAR Pivotal Trial Implants Complete Target for FDA approval & US commercial launch • Inspire is a spin out from Medtronic • 170+ global implants conducted at 25+ global clinical sites • Commercially available in Europe (CE Mark received in 2010) • The Inspire system is for Investigational Use Only in the United States • Mark Christopherson is an employee of Inspire Medical Systems
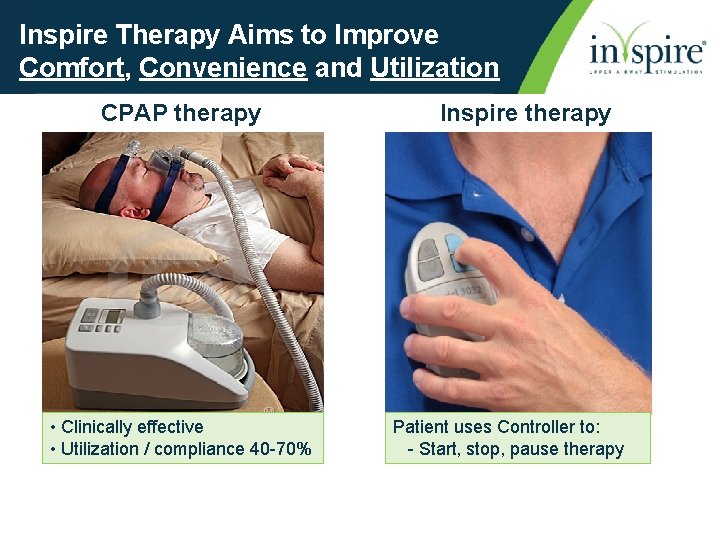
Inspire Therapy Aims to Improve Comfort, Convenience and Utilization CPAP therapy • Clinically effective • Utilization / compliance 40 -70% Inspire therapy Patient uses Controller to: - Start, stop, pause therapy
- Slides: 17