A Seminar on VALIDATION OF COMPRESSED AIR CONTENT
























- Slides: 33

A Seminar on VALIDATION OF COMPRESSED AIR

CONTENT v Introduction v How Compressed Air System(CAS) works? v Test functions and Acceptance criteria v Validation protocol : ü Installation Qualification (IQ) ü Operational Qualification (OQ) ü Performance Qualification (PQ) v Applications v How to gain efficiencies and power savings? v References 2

Introduction Ø What is compressed air? § Commonly called Industry's Fourth Utility § Air that is condensed and contained at a pressure that is greater than the atmosphere § The process takes a given mass of air, which occupies a given volume of space, and reduces it into a smaller space. In that space, greater air mass produces greater pressure. The pressure comes from this air trying to return to its original volume § It is used in many different manufacturing operations. A typical compressed air system operating at 100 psig (7 bar) will compress the air down to 1/8 of its original volume. (figure CA 1 -1) 3

• Compressed air is an important component of pharmaceutical manufacturing facilities • It provides many of the air types necessary for a manufacturing facility to function, including: breathing air , motive air for machines , process air , analytical air and Product Direct Impact , or "c. GMP" air • The application for which the compressed air will be used will dictate the level of air quality that is appropriate for use • There are two main categories of applications: Direct Impact Systems and Indirect Impact System 4

Direct Impact Systems : • The systems will have direct contact with the product • The system will provide an excipient, or produce an ingredient or solvent • The system will be used in cleaning or sterilizing • The system will preserve product status • The system will produce data which is used to accept or reject the product • The system will be a process control system that can affect product quality and there will be no system for independent verification of control system performance 5

Indirect Impact or No Impact Systems : • The system will not contact the product or materials that ultimately become part of the product • The systems are generally site or building systems and are not tailored specifically to aseptic manufacturing facilities • The systems will deal with a side activity of the manufacturing process (such as waste disposal) 6

How the Compressed Air System(CAS) works? • Each component in a typical system helps to deliver clean, dry, compressed air that’s free of pressure fluctuations at its point of use • If any component is working inefficiently, the system’s performance suffers and operating costs rise • Like any high-pressure system, they are prone to leaks or other failures that can result in lower performance 7

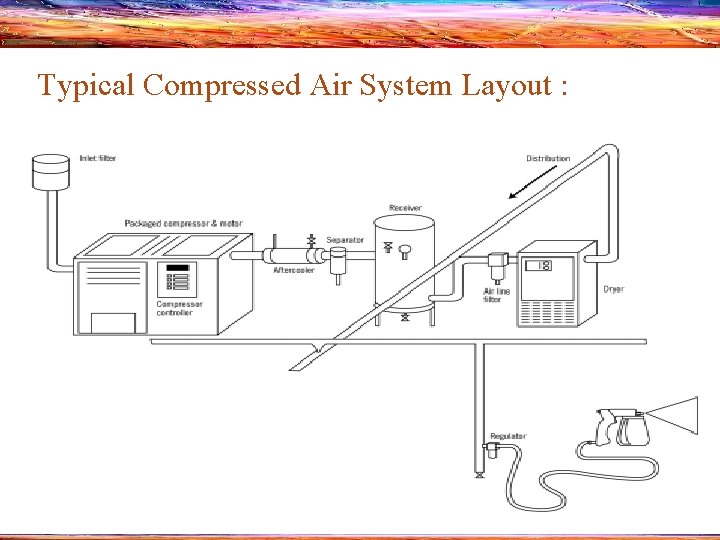
Typical Compressed Air System Layout : 8

Components of the CAS system • Inlet filter: Removes particles from the air entering the compressor • Compressor : Compresses air to a small volume, increasing the pressure (Reciprocating compressors are frequently used) • Motor : Drives the compressor • Compressor Controller : Directs the compressor’s output and it may be microprocessor, 9 electromechanical or pneumatically based

• Aftercooler : Compression leaves the air hot and wet. The aftercooler lowers the temperature of the air leaving the compressor and removes water that condenses as the air cools • Separator : Removes liquids from the compressed air • Receiver : Stores a large reserve of compressed air to maintain a smooth flow to the plant • Air line Filter : Removes solids and liquids from the compressed air stream. Can be placed throughout the system 10

• Dryer : - Helps to eliminate any remaining moisture in the compressed air by using either a refrigerated condenser or a desiccant - Refrigerated condensers cool the air to condense water vapours into a liquid that is then drained from the system. Desiccants are powders or gels that remove water by absorbing it • Condensate Trap : - Collects and discharges liquid that condenses out of the air stream - Integral part of aftercoolers, dryers and separators 11

• Distribution Piping : Links the components. It distributes the air from a main header to branch lines and sub headers to drop points connected to individual tools • Pressure regulator : Controls air pressure and flow at individual points of use 12

Test Functions : 1. Perform Installation Qualification 2. Perform general operational controls verification testing 3. Operate system throughout the range of operating design specifications or range of intended use 4. Verify that the compressed air system is capable of supplying pressurized compressed air to all use points. Perform an operational test of the distribution system and pressure regulators by monitoring the pressure output at the 13 respective use points

5. Perform a capacity test to verify that the system is capable of supplying the required gas, pressure, and flow rate at each use point 6. Verify that in-line filters are integrity tested. Confirm that all documentation clearly indicates acceptable test results 7. Perform dew point measurement 8. Perform hydrocarbon content measurement 9. Perform viable particulate count, microbiological at critical use points after sterile filters (refer to Federal Standard 209 E) 14

10. Identification of oxygen content 11. Record the range of all process or equipment parameters (set points, flow rates, timing sequences, concentrations, etc. ) verified during Operational and Performance Qualifications testing 15

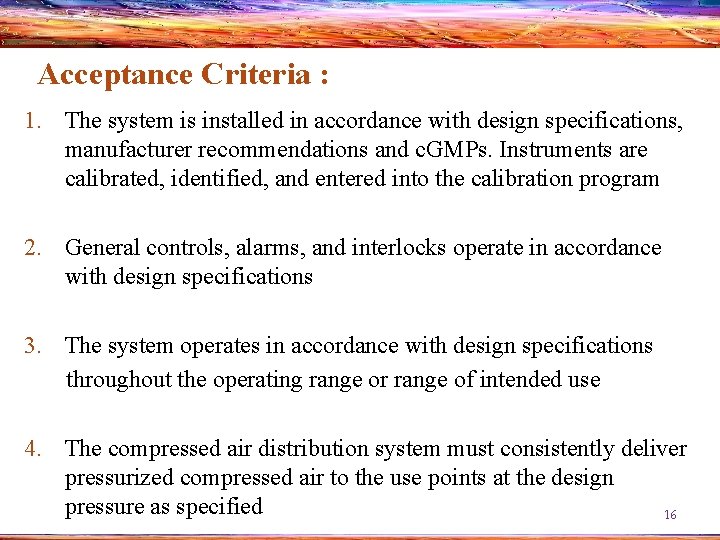
Acceptance Criteria : 1. The system is installed in accordance with design specifications, manufacturer recommendations and c. GMPs. Instruments are calibrated, identified, and entered into the calibration program 2. General controls, alarms, and interlocks operate in accordance with design specifications 3. The system operates in accordance with design specifications throughout the operating range or range of intended use 4. The compressed air distribution system must consistently deliver pressurized compressed air to the use points at the design pressure as specified 16

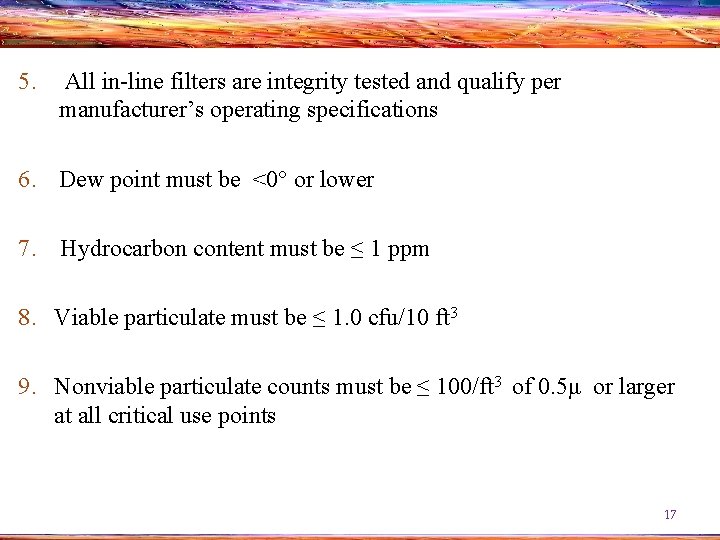
5. All in-line filters are integrity tested and qualify per manufacturer’s operating specifications 6. Dew point must be <0° or lower 7. Hydrocarbon content must be ≤ 1 ppm 8. Viable particulate must be ≤ 1. 0 cfu/10 ft 3 9. Nonviable particulate counts must be ≤ 100/ft 3 of 0. 5μ or larger at all critical use points 17

Method for Dew point and Oil content • As per Air Quality Standards ISO 8573 and Filter Standard ISO 12500 for compressed air there are nine part to it as follows : Part 1 - Contaminants and Purity classes Part 2 - Test methods for aerosol oil content Part 3 - Test methods for the measurement of humidity Part 4 - Test methods for solid particle content Part 5 - Determination of oil vapor and organic solvent content Part 6 - Test methods for gaseous contaminant content Part 7 - Test method for viable microbiological contaminant content Part 8 - Test methods for solid particle content by mass concentration Part 9 - Test methods for liquid water content 18

Validation Protocol : • It includes : ü ü Installation Qualification Operational Qualification Performance Qualification Final Report 19

Installation Qualification (IQ) • This section establishes documented verification that key aspects of equipment adhere to approve design intentions and recommendation of manufacturer have been suitably consider • In addition to the common requirements outlined in the "General" section, the following are required for Distribution systems • The piping should be supported, labeled, and sloped to drain completely 20

Contd… • Materials of construction will vary with gas type, and must agree with specification • Verify that in-line filters can be integrity tested • Verify that the systems have been thoroughly cleaned and dried before operation 21

Operational Qualification • This section Establishes that there is a documented verification that the installed system functions as a specified and that there is a sufficient documentary evidence to demonstrate this • The OQ protocol will outline tests to study capacity and pressure during estimated minimum and maximum use 22

Contd… • All use points supply the specified pressure prior to any pressure reducing valves or equipment • All use point supply the volume of gas as specified • Each peak load use point as specified by use or equipment 23

Performance Qualification • This section gives documented verification that the equipment performance in its normal operating environment is consistently exactly as specified in User Requirement Specification (URS) • Testing will include viable and total particulate counts, dew point, hydrocarbon analysis, and purity analysis • Each point of use will be tested at least three times over 10 working days. Every use point of the system must be tested several times over the course of the study. 24

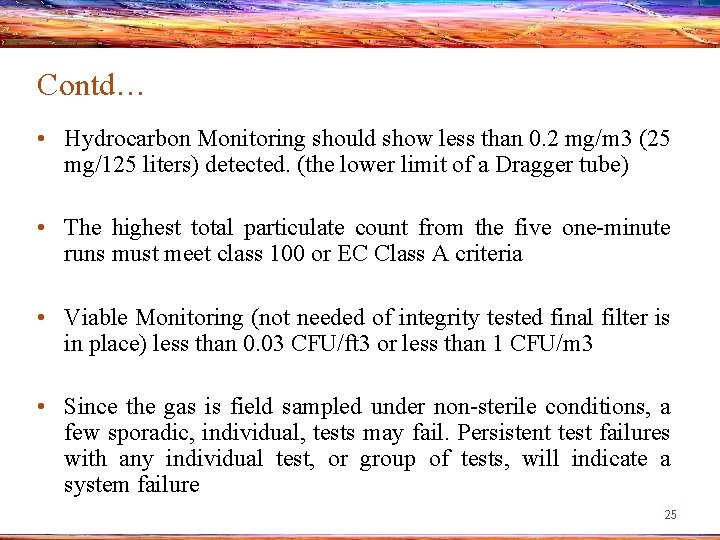
Contd… • Hydrocarbon Monitoring should show less than 0. 2 mg/m 3 (25 mg/125 liters) detected. (the lower limit of a Dragger tube) • The highest total particulate count from the five one-minute runs must meet class 100 or EC Class A criteria • Viable Monitoring (not needed of integrity tested final filter is in place) less than 0. 03 CFU/ft 3 or less than 1 CFU/m 3 • Since the gas is field sampled under non-sterile conditions, a few sporadic, individual, tests may fail. Persistent test failures with any individual test, or group of tests, will indicate a system failure 25

Contd… • The dew point of compressed air less than or equal to -10°C, or less than the lowest temperature to which the system is exposed • Identity and Purity (Nitrogen) Not less than 99. 0% nitrogen by volume. Not more than 0. 001% Carbon Monoxide. No appreciable odor • Identity and Purity (Oxygen) Not less than 99. 0% Oxygen by volume. No appreciable odor 26

Final Report • Depending on IQ , OQ and PQ data final report is made and that will indicate whether your system is validated or not 27

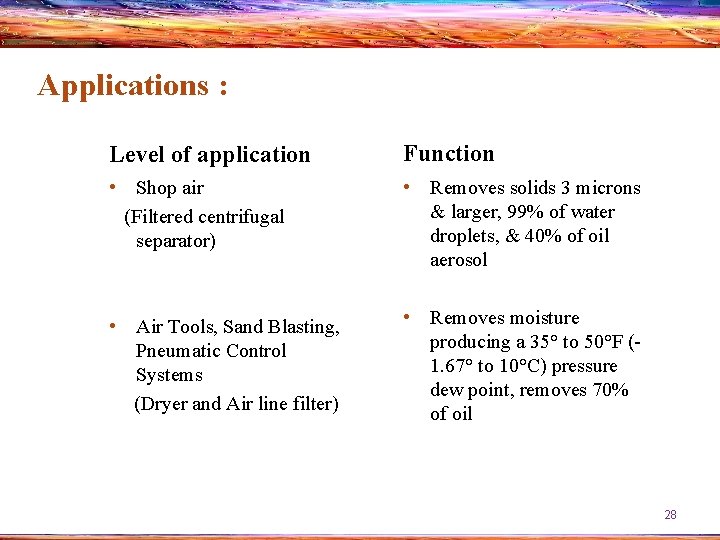
Applications : Level of application Function • Shop air (Filtered centrifugal separator) • Removes solids 3 microns & larger, 99% of water droplets, & 40% of oil aerosol • Air Tools, Sand Blasting, Pneumatic Control Systems (Dryer and Air line filter) • Removes moisture producing a 35° to 50°F (1. 67° to 10°C) pressure dew point, removes 70% of oil 28

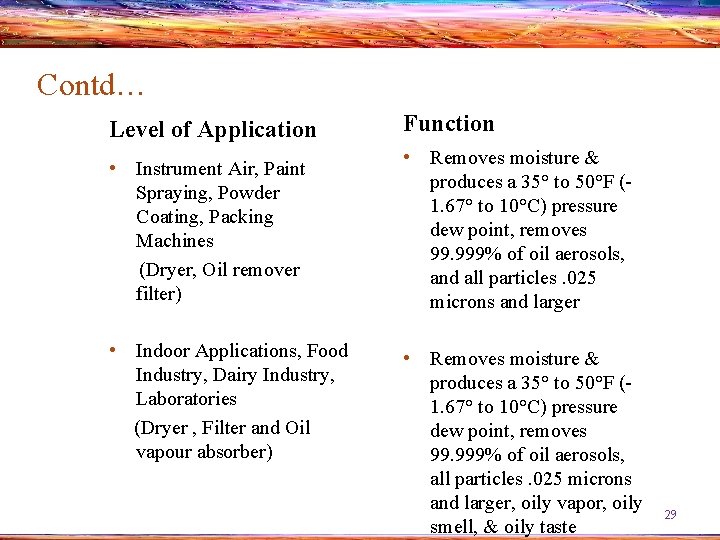
Contd… Level of Application Function • Instrument Air, Paint Spraying, Powder Coating, Packing Machines (Dryer, Oil remover filter) • Removes moisture & produces a 35° to 50°F (1. 67° to 10°C) pressure dew point, removes 99. 999% of oil aerosols, and all particles. 025 microns and larger • Indoor Applications, Food Industry, Dairy Industry, Laboratories (Dryer , Filter and Oil vapour absorber) • Removes moisture & produces a 35° to 50°F (1. 67° to 10°C) pressure dew point, removes 99. 999% of oil aerosols, all particles. 025 microns and larger, oily vapor, oily smell, & oily taste 29

Contd… Level of Application Function • Breathing Air (continuous or portable) • Removes harmful compressed air contaminants and will produce Grade D breathing air 30

How to get efficiency and power savings? • Establish regular maintenance programme • Hunt for air leaks • Educate your plant staff and emphasizing the importance of monitoring the line • Check system operating pressure 31

References Ø Agalloco James, Carleton J. Fredric “Validation of Pharmaceutical Processes”; Third edition Ø Syed Imitiaz Haider, Pharmaceutical Master Validation Plan, The ultimate guide to FDA , GMP and GLP compliance Ø Syed Imtiaz Haider, “Validation Standard Operating Procedure”, 2 nd Edition Ø www. usvalidation. com Ø http: //www. validationwhat. com/compressed-air-quality. html Ø http: //validationworld. com 32

THANK YOU 33
 Compressed air validation
Compressed air validation Compressed air supercharging
Compressed air supercharging Compressed air energy storage
Compressed air energy storage Compressed air business
Compressed air business Wilkerson desiccant air dryer
Wilkerson desiccant air dryer Pt tanah air sentosa
Pt tanah air sentosa What is esp
What is esp Static content vs dynamic content
Static content vs dynamic content Fertilisers
Fertilisers Why is gas easier to compress than a liquid or a solid
Why is gas easier to compress than a liquid or a solid Visual search
Visual search Compressed language in poetry
Compressed language in poetry 29 cfr 1910 compressed gas cylinder storage
29 cfr 1910 compressed gas cylinder storage Compressed gas training powerpoint
Compressed gas training powerpoint Compressed rvc foam
Compressed rvc foam Compressed modernity definition
Compressed modernity definition Osha psm 1910
Osha psm 1910 Pereopods
Pereopods Sugar coating
Sugar coating Answers
Answers Compressed gas definition
Compressed gas definition Hypodermic tablets definition
Hypodermic tablets definition Compressed trie
Compressed trie Yuvpak compressed fractal image
Yuvpak compressed fractal image Compressed gas hazard
Compressed gas hazard Vertical stretch and compression
Vertical stretch and compression Calendering moulding process
Calendering moulding process Compressed liquid water
Compressed liquid water A swirling center of low air pressure is called
A swirling center of low air pressure is called Air payau adalah campuran…. *
Air payau adalah campuran…. * Can you feel it in the air in the air
Can you feel it in the air in the air Pelarut protonik
Pelarut protonik Air masses & frontswhat is an air mass?
Air masses & frontswhat is an air mass? The boundary between two adjacent air masses is called
The boundary between two adjacent air masses is called