A SAS and JMP Clinical Template for Central


















- Slides: 41

A SAS® and JMP® Clinical Template for Central Statistical Monitoring Discovery Summit, Amsterdam, March 15 -17, 2016 Egbert A. van der Meulen

Agenda • About Ferring … Agenda • What is Central Statisticial Monitoring (CSM) ? • WHO wa ARE • What should be top-priorities? • Our SAS environment; • How are we presently implementing CSM • A • demonstration of the resulting SAS JMP Clinical template for Some stories, perceptions and observed trends CSM (beta version) • What wa started off with • Practical aspects • What ARE typical conceptual issues with CSM • Questions • What ARE wa doing now

About Ferring

Ferring YESTERDAY Ferring’s foundation 1909– 1997 Frederik Paulsen Senior Frederik Paulsen 1950: Founded the first research 1988: Dr Paulsen’s son took company to synthesise pituitary hormones on an industrial scale. 4 1950 - over as Ferring Group CEO and expanded the business into more than 100 countries. Today Frederik Paulsen is Chairman of the Board.

Ferring TODAY Key Therapeutic areas Ferring’s R&D activities are focused on the development of first-in-class therapeutic peptides and proteins in our key therapeutic areas Reproductive Health 5 Gastroenterology Urology Other

Ferring TODAY Fully integrated R&D organization with ~600 scientists IPC DK Development Hub FCT UK Parsippany Development Hub FGLAG Ferring China San Diego FICSA IPC India Research site BTG Israel 6 Ferring Japan


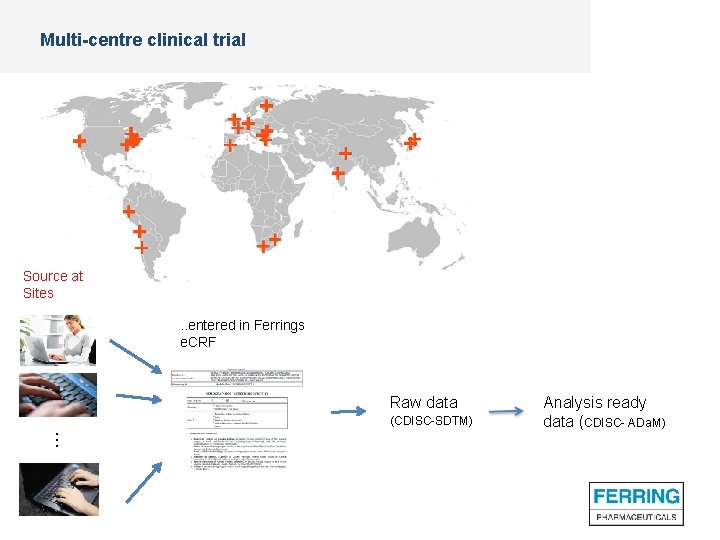
Multi-centre clinical trial Source at Sites . . entered in Ferrings e. CRF Raw data (CDISC-SDTM) Analysis ready data (CDISC- ADa. M) …

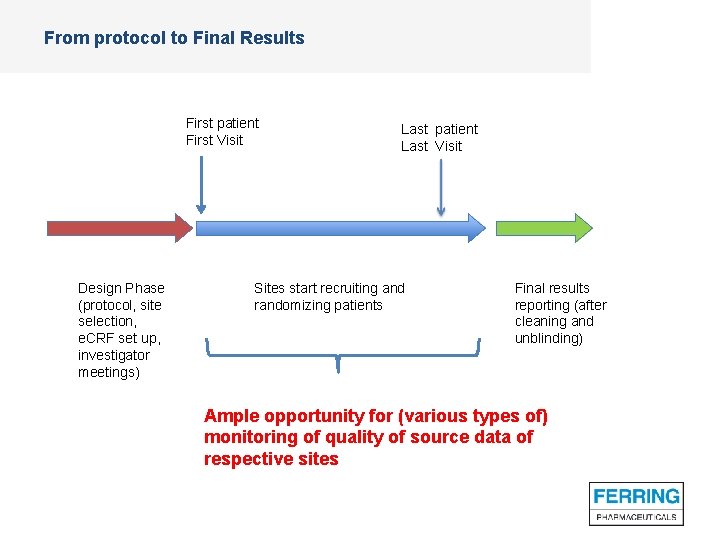
From protocol to Final Results First patient First Visit Design Phase (protocol, site selection, e. CRF set up, investigator meetings) Last patient Last Visit Sites start recruiting and randomizing patients Final results reporting (after cleaning and unblinding) Ample opportunity for (various types of) monitoring of quality of source data of respective sites

What is CSM, Central Statistical Monitoring ?

Quotes from FDA 2013 Guidance on Risk-Based Monitoring * “ Conduct statistical analyses to identify data trends not easily detected by onsite monitoring, such as … checks for unusual distribution of data within and between study sites, such as too little variance … “… routine review of submitted data to identify and follow-up on missing data, inconsistent data, data outliers, and … that may be indicative of systemic or significant errors in data collection and reporting at a site” “… focusing on the most critical data elements, are more likely than routine visits to all clinical sites and 100% data verification to ensure subject protection and overall study quality” * FDA Guidance for Industry: Oversight of Clinical Investigations – A Risk-Based Approach to Monitoring, August 2013. http: //www. fda. gov/downloads/Drugs/. . . /Guidances/UCM 269919. pdf

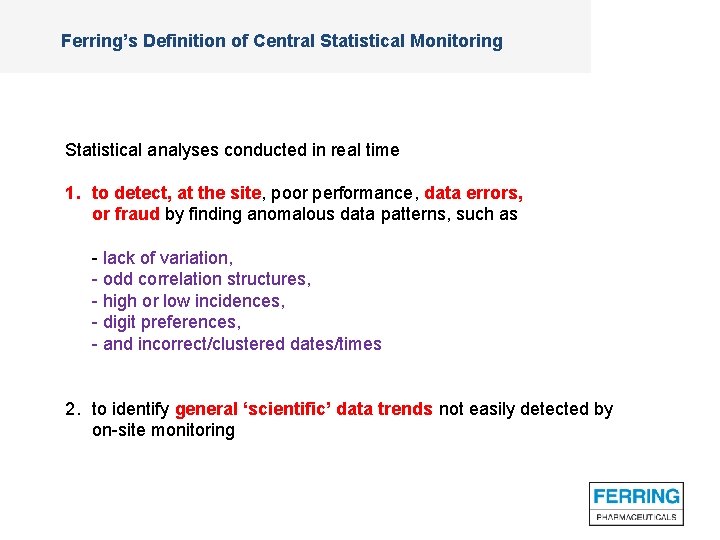
Ferring’s Definition of Central Statistical Monitoring Statistical analyses conducted in real time 1. to detect, at the site, poor performance, data errors, or fraud by finding anomalous data patterns, such as - lack of variation, - odd correlation structures, - high or low incidences, - digit preferences, - and incorrect/clustered dates/times 2. to identify general ‘scientific’ data trends not easily detected by on-site monitoring

What should be top-priorities: further summarized 1. Fraud by particular sites 2. Generally ’poor’ recruitment by many sites


Source: Statistics and Truth, Putting Chance to Work by C. R. Rao (1989)

Poor Recruitment as reflected by artifically inflated baseline values used for eligibility

An observed trend I worry about slightly CGRI RACT Ri sk Ind ica R SD RM P to rs IQRMP Th res Qb D ho lds Need a tool that allows this: ”SAS JMP Clincial template” Careful not to turn into burocrates The core should be true (Oxford –educated) detective work Source: Trans. Celerate PP

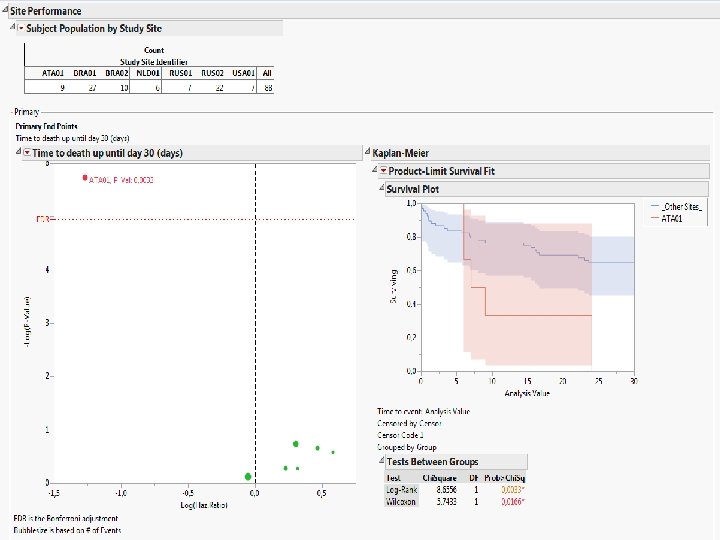
How are we presently implementing CSM • Clinical trials, Regulatory Submission, Publications: SDD 4. 5 (Clin Data Mngt, Programming, and Biostats) • Trial Design: ADDPLAN, SAS (within SDD), R • Central Statistical Monitoring: SAS JMP Clinical Jointly with SAS Denmark have build a template that (1) Allows evaluating site performance using statistical inference (p-values!) (2) Enables evaluation overall performance, particularly with regard to Recruitment Quality Kasper Munck Business Delivery Manager SAS Institute A/S, Denmark

Practical Aspects • Requires instant availability of SDTM/ADa. M (from FPFV onward). Having e. CRF and CDISC standards this is doable • Additional routine role for trial statistician: data-mine the data from CSM perspective and discuss during clinical trial team meetings while trial is ongoing • Have to dealing with ”Ongoingness” of the data • Document: standard (push-on-one-button ) auto-reporting during the meeting • Define follow-up action as needed (e. g. re-train site, site- audit, stop trial, etc) • Requires new SOP(s) on CSM

Thank You Discovery Summit, Amsterdam, March 15 -17, 2016 Egbert A. van der Meulen

Questions

BACK UP

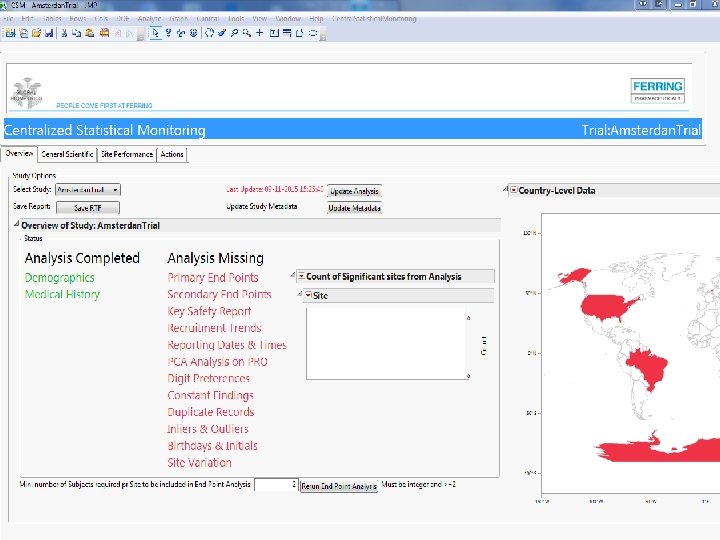
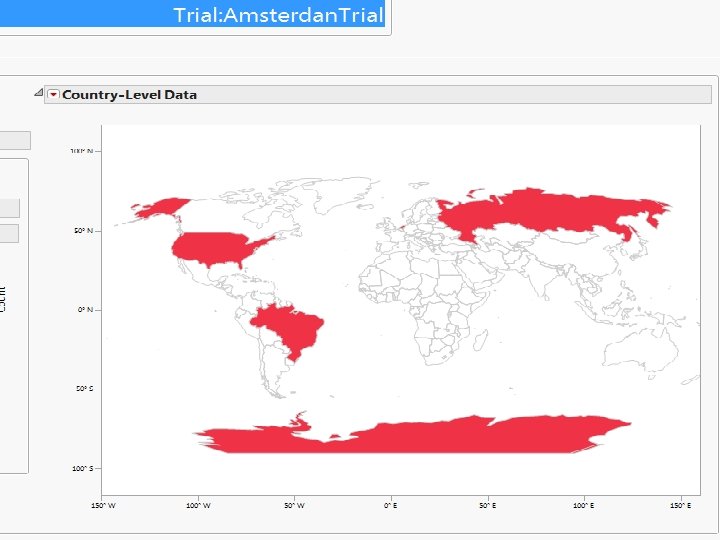
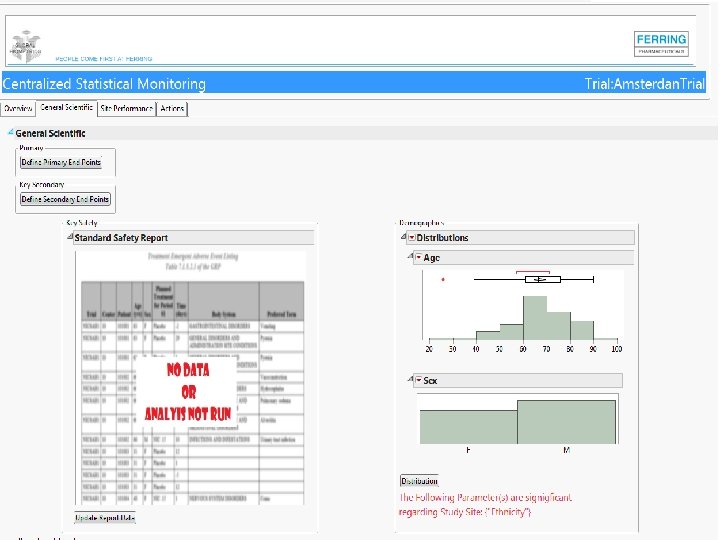
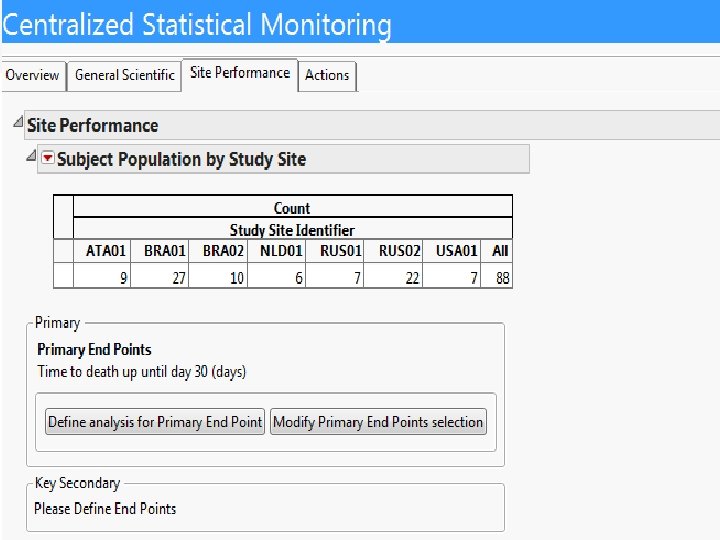
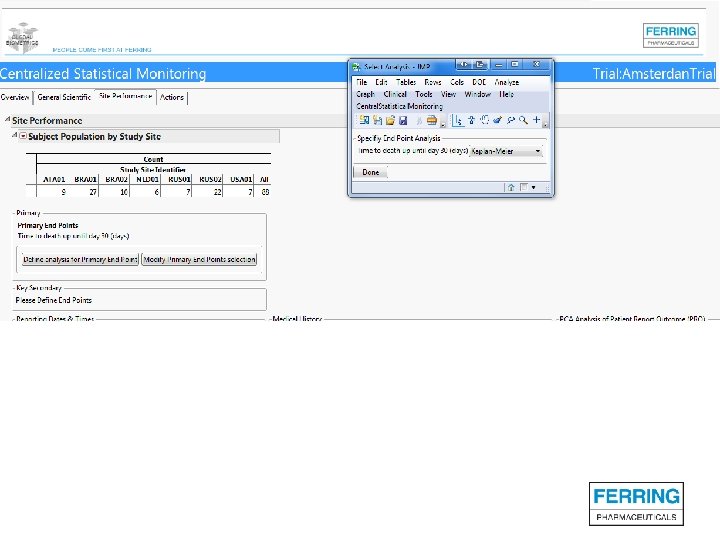
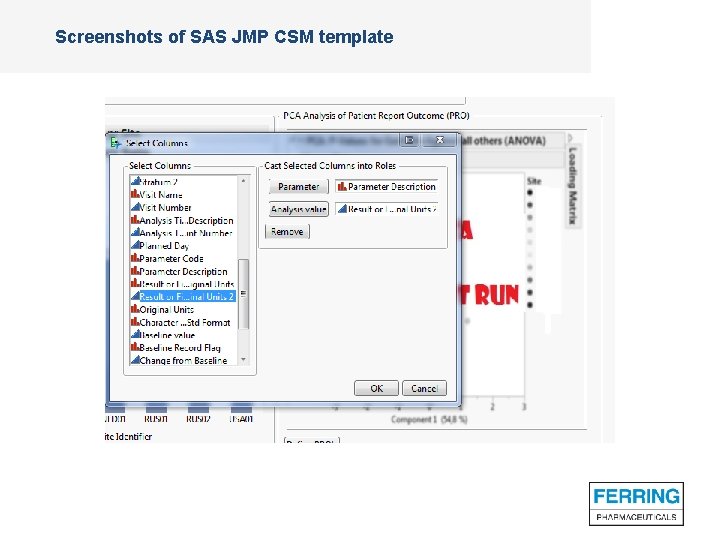
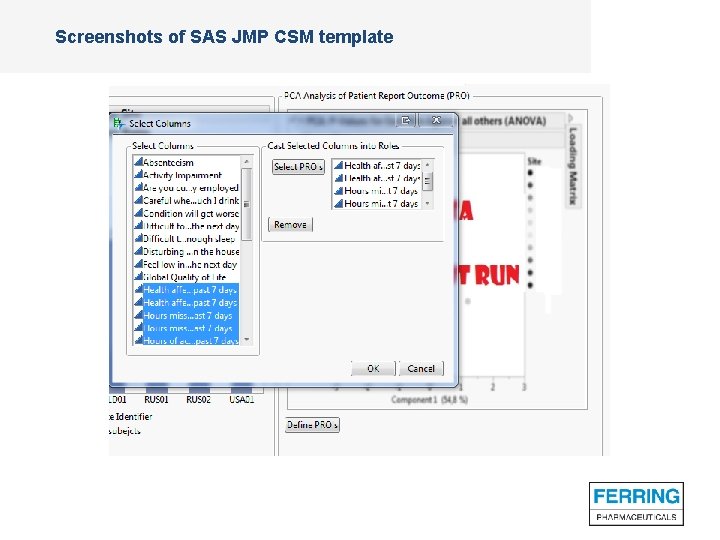
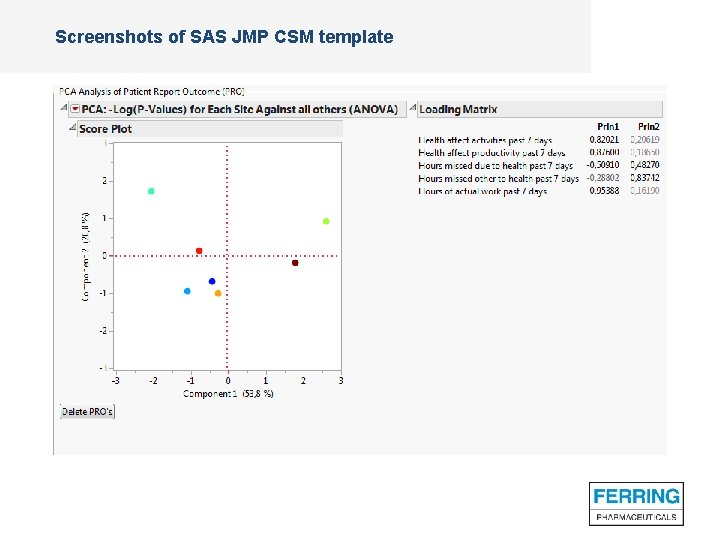
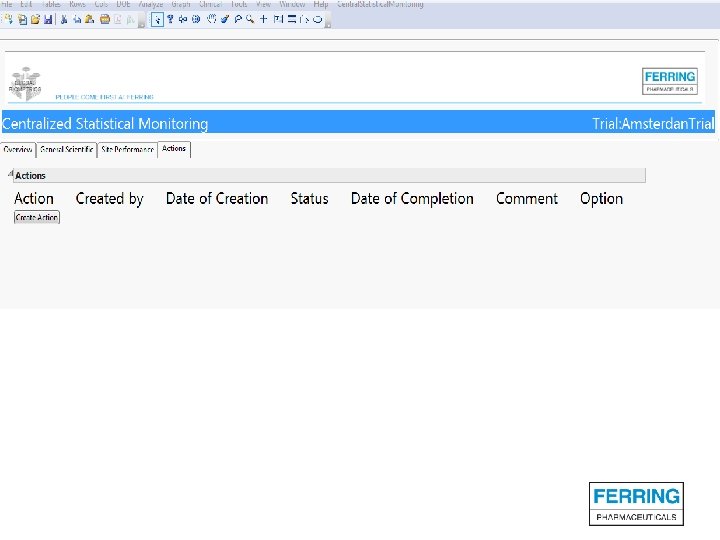
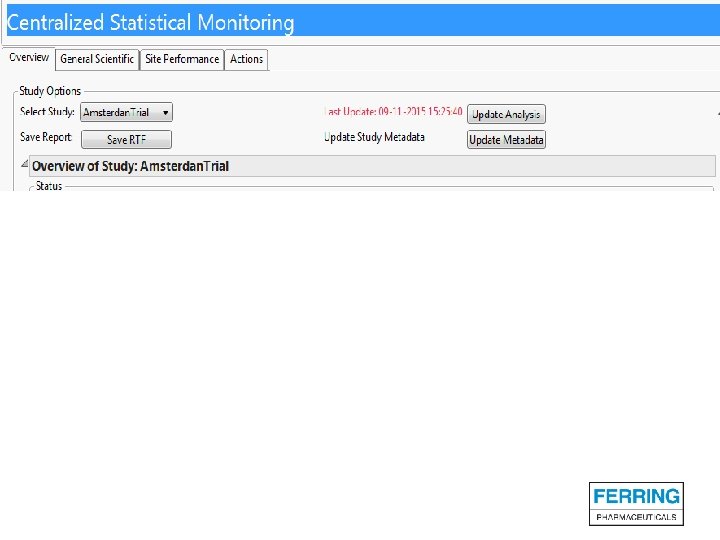
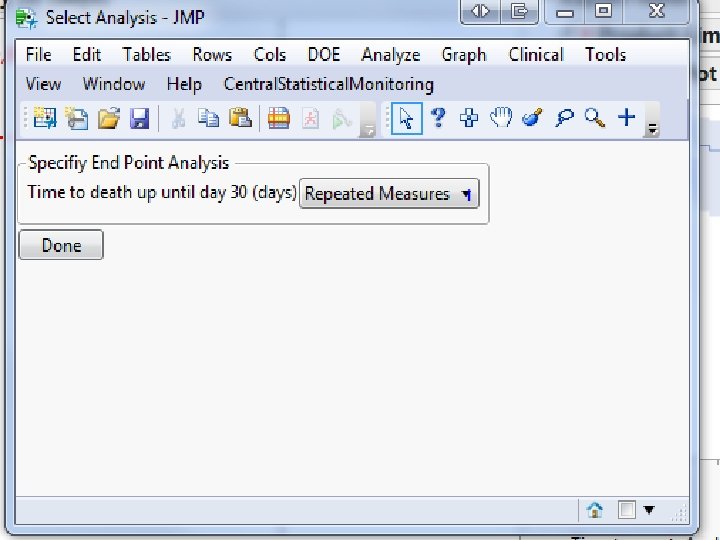
Screenshots of SAS JMP CSM template

Screenshots of SAS JMP CSM template

Screenshots of SAS JMP CSM template

Screenshots of SAS JMP CSM template

Screenshots of SAS JMP CSM template

Screenshots of SAS JMP CSM template

Screenshots of SAS JMP CSM template

Screenshots of SAS JMP CSM template

Screenshots of SAS JMP CSM template

Screenshots of SAS JMP CSM template

Screenshots of SAS JMP CSM template

Screenshots of SAS JMP CSM template

Screenshots of SAS JMP CSM template

Ad –hoc Programming in R (instead of SAS) Example (CS 36, IPSS items ): correlation matrix plots by site

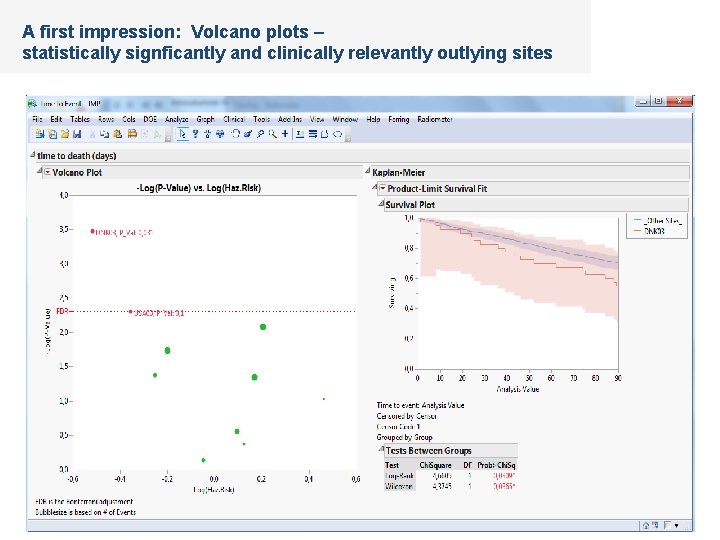
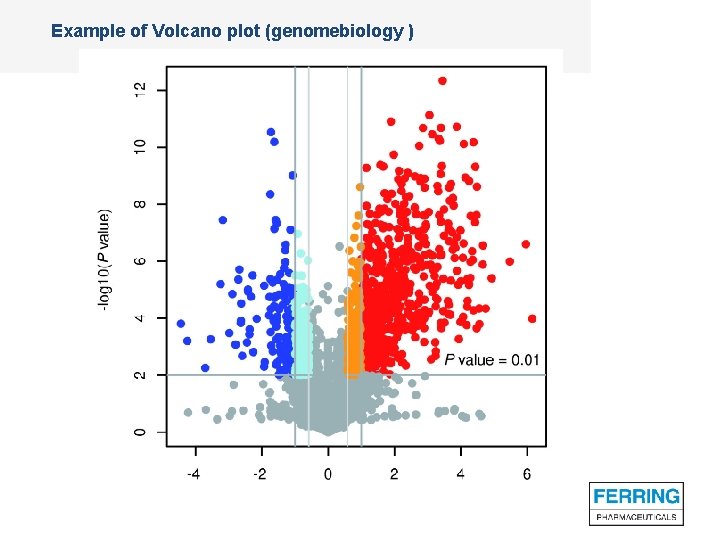
A first impression: Volcano plots – statistically signficantly and clinically relevantly outlying sites

Example of Volcano plot (genomebiology )

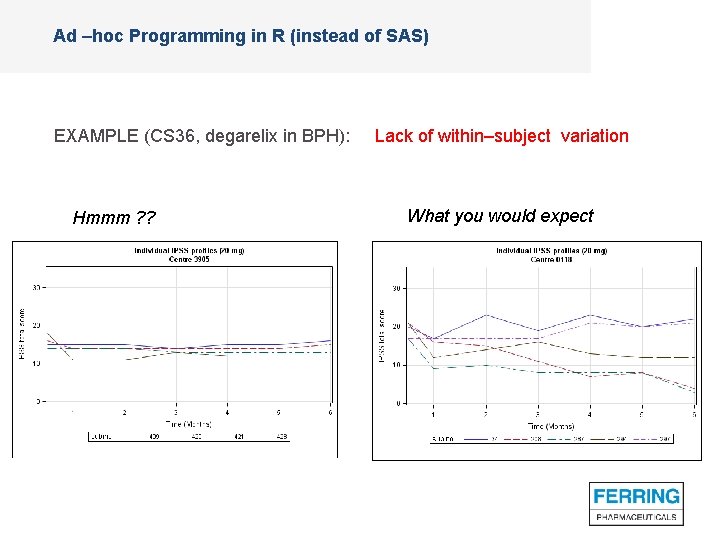
Ad –hoc Programming in R (instead of SAS) EXAMPLE (CS 36, degarelix in BPH): Lack of within–subject variation Hmmm ? ? What you would expect

A few stories from the past Hector, from ”Meggicco” From a Ph III, Global HRT trial

Hector ’s method of ”adhering to the ITT principle (complete data)”