A sample of an aldehyde has been extracted

A sample of an aldehyde has been extracted in almost pure form (95%) from cucumber. You are provided with a 1 H NMR spectrum (500 MHz; CDCl 3) of the sample. The numbers above the peaks on the proton spectrum are the relative integrals of the peaks of the major component. You are also provided with COSY (showing the couplings between the peaks of the major component) and HSQC spectra. Identify the major component and assign the spectra.
(3) (2)(2) (1) 9 8 7 (1)(1) 6 5 4 3 2 1 ppm


HSQC – Cucumber aldehyde
![You are provided with NMR data for a sample of 5 HIndeno[1, 2 -b]pyridin-5 You are provided with NMR data for a sample of 5 HIndeno[1, 2 -b]pyridin-5](http://slidetodoc.com/presentation_image_h/bd1624cebf19252642c20ab816fa1d14/image-5.jpg)
You are provided with NMR data for a sample of 5 HIndeno[1, 2 -b]pyridin-5 -one at 500 MHz in CDCl 3. Assign the carbon spectrum.

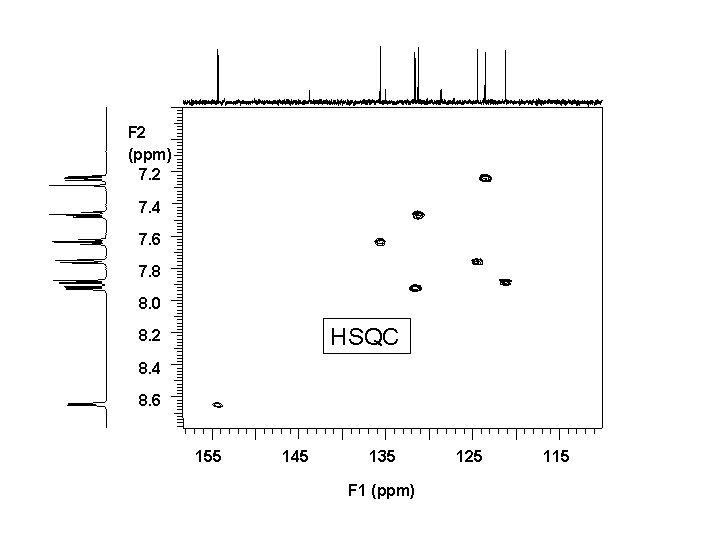
F 2 (ppm) 7. 2 7. 4 7. 6 7. 8 8. 0 HSQC 8. 2 8. 4 8. 6 155 145 135 F 1 (ppm) 125 115

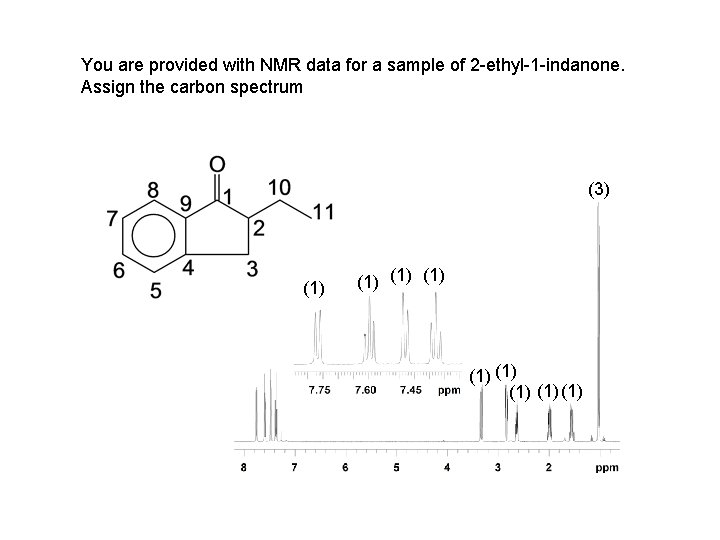
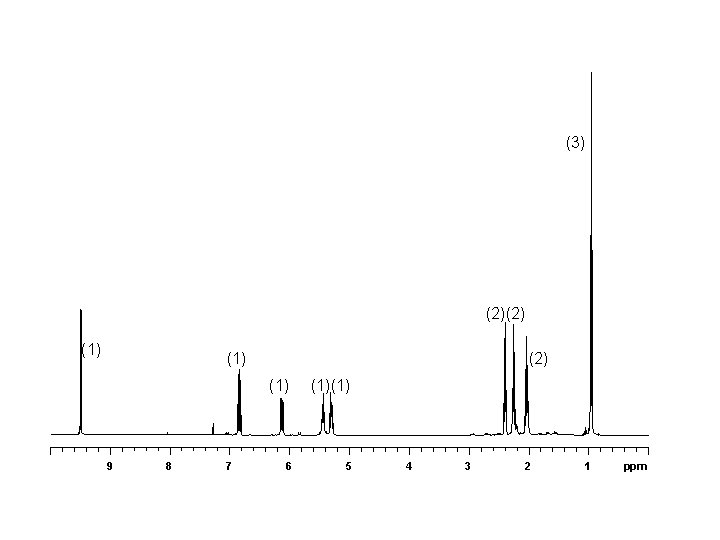
You are provided with NMR data for a sample of 2 -ethyl-1 -indanone. Assign the carbon spectrum (3) (1) (1) (1)



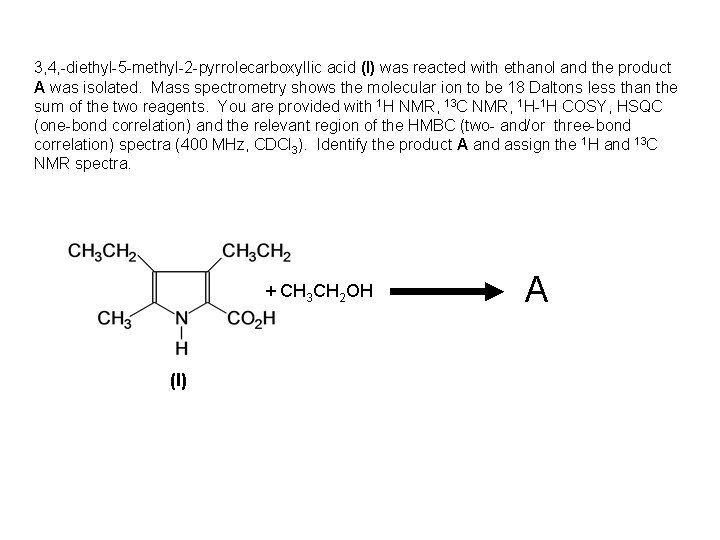
3, 4, -diethyl-5 -methyl-2 -pyrrolecarboxyllic acid (I) was reacted with ethanol and the product A was isolated. Mass spectrometry shows the molecular ion to be 18 Daltons less than the sum of the two reagents. You are provided with 1 H NMR, 13 C NMR, 1 H-1 H COSY, HSQC (one-bond correlation) and the relevant region of the HMBC (two- and/or three-bond correlation) spectra (400 MHz, CDCl 3). Identify the product A and assign the 1 H and 13 C NMR spectra. + CH 3 CH 2 OH (I) A

COSY

HSQC

HMBC
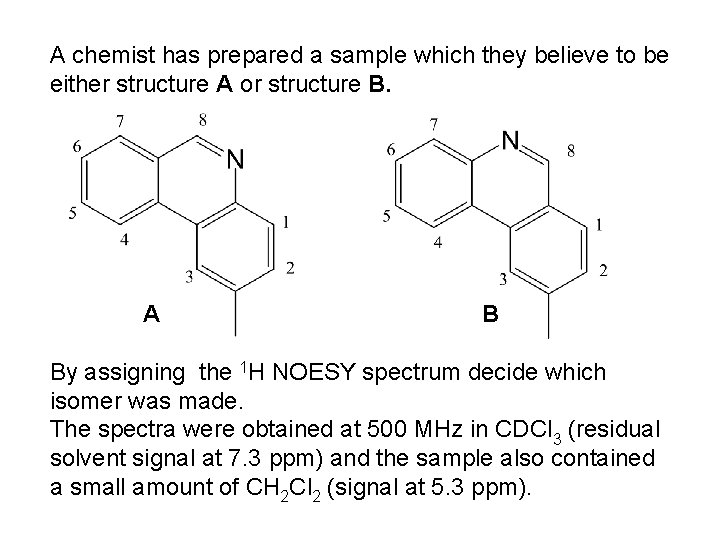
A chemist has prepared a sample which they believe to be either structure A or structure B. A B By assigning the 1 H NOESY spectrum decide which isomer was made. The spectra were obtained at 500 MHz in CDCl 3 (residual solvent signal at 7. 3 ppm) and the sample also contained a small amount of CH 2 Cl 2 (signal at 5. 3 ppm).


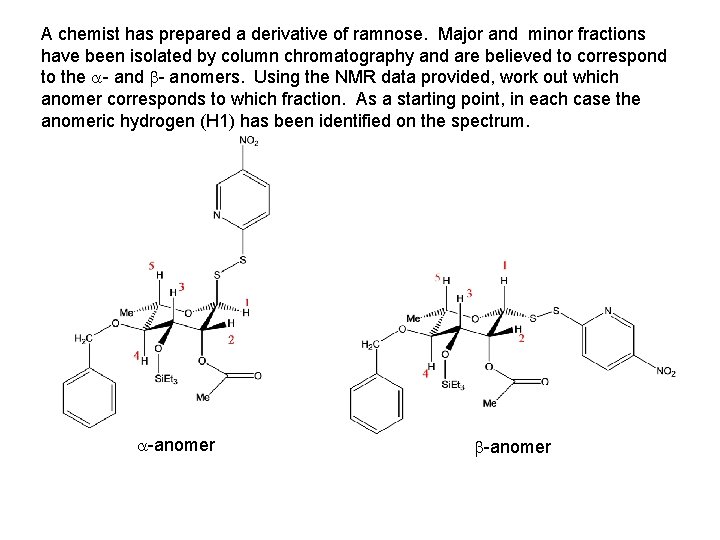
A chemist has prepared a derivative of ramnose. Major and minor fractions have been isolated by column chromatography and are believed to correspond to the - and - anomers. Using the NMR data provided, work out which anomer corresponds to which fraction. As a starting point, in each case the anomeric hydrogen (H 1) has been identified on the spectrum. -anomer
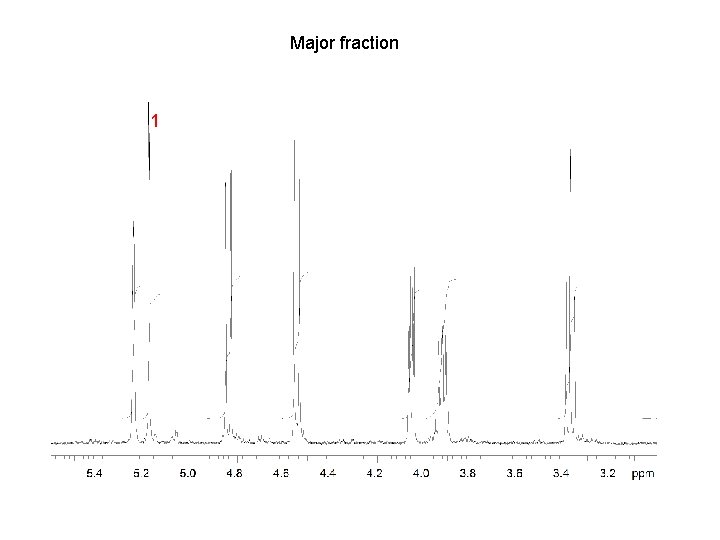
Major fraction 1

Minor fraction 1

Major fraction - COSY

Major fraction - NOESY

Minor fraction - COSY

Minor fraction - NOESY

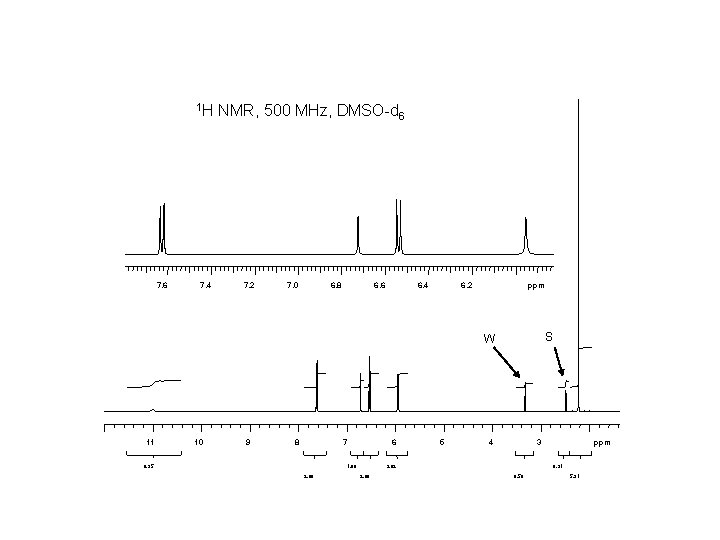
1 H 7. 6 7. 4 NMR, 500 MHz, DMSO-d 6 7. 2 7. 0 6. 8 6. 6 6. 4 6. 2 ppm S W 11 10 9 8 7 0. 95 6 1. 00 2. 00 5 4 3 2. 02 2. 06 ppm 0. 91 0. 56 5. 91
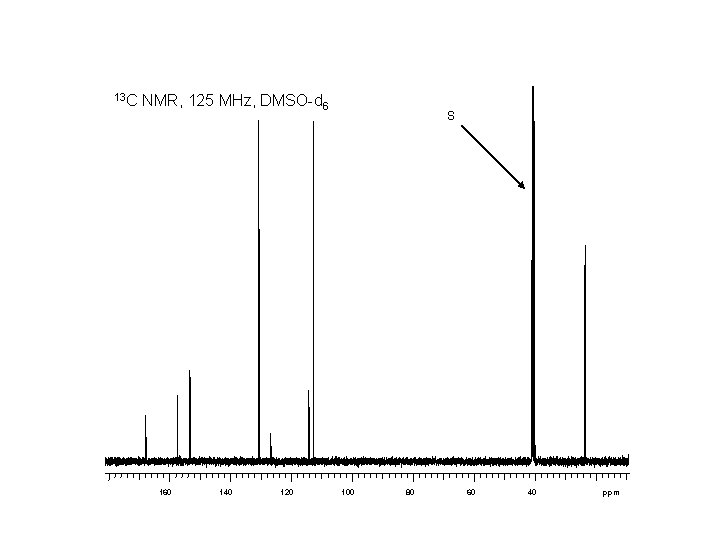
13 C NMR, 125 MHz, DMSO-d 6 160 140 120 S 100 80 60 40 ppm
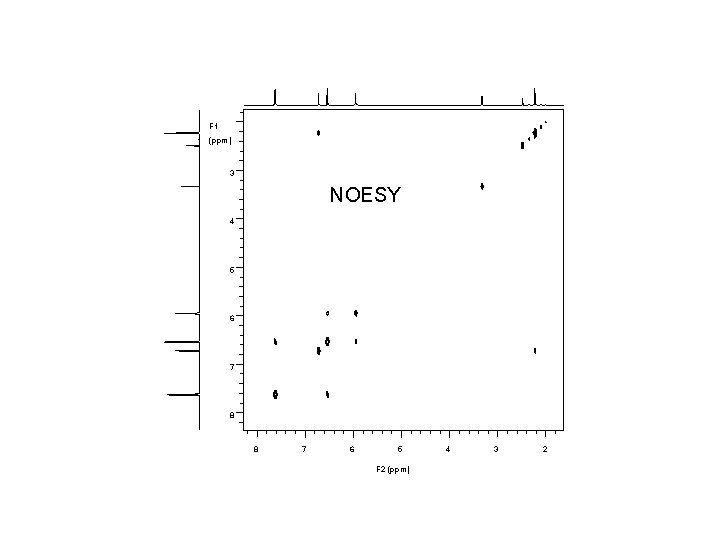
F 1 (ppm) 3 NOESY 4 5 6 7 8 8 7 6 5 F 2 (ppm) 4 3 2
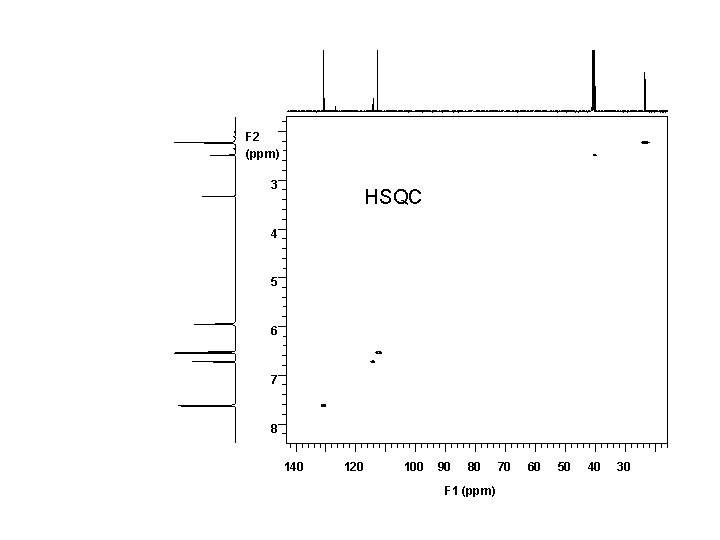
F 2 (ppm) 3 HSQC 4 5 6 7 8 140 120 100 90 80 F 1 (ppm) 70 60 50 40 30
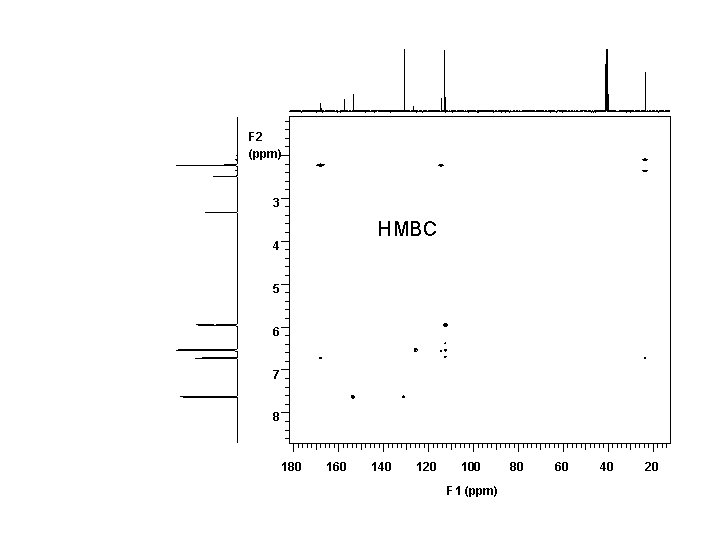
F 2 (ppm) 3 HMBC 4 5 6 7 8 180 160 140 120 100 F 1 (ppm) 80 60 40 20
- Slides: 32