A Randomized Trial of Supplemental Parenteral Nutrition in




































































- Slides: 104

A Randomized Trial of Supplemental Parenteral Nutrition in Under and Over Weight Critically Ill Patients: The TOP UP Trial April 12 th 2012 Study Sponsor: Dr. Daren Heyland Project Leader: Rupinder Dhaliwal Project Assistant: Roger Leung Clinical Evaluation Research Unit

Protocol: Version April 11 th 2012

Background & Objectives

• Point prevalence survey of nutrition practices in ICU’s around the world conducted Jan. 27, 2007 • Enrolled 2772 patients from 158 ICU’s over 5 continents • Included ventilated adult patients who remained in ICU >72 hours

What study patients actually received? • Average Calories in all groups: – 1034 kcals and 47 gm of protein Result: • Average caloric deficit in Lean Pts: – 7500 kcal/10 days • Average caloric deficit in Severely Obese: – 12000 kcal/10 days


ICU patients are not all created equal…should we expect the impact of nutrition therapy to be the same across all patients?

TOP UP Trial: Hypothesis Increased early energy and protein delivery with PN+EN to underweight (BMI < 25) and obese (BMI ≥ 35) critically ill patients will result in improved survival at 60 day versus standard EN alone

Objectives • Perform an initial multi-center pilot study in Canada, USA, France & Belgium in 160 patients to demonstrate feasibility • Assuming feasibility, large-scale 2000 patient multicenter, multinational trial will be undertaken

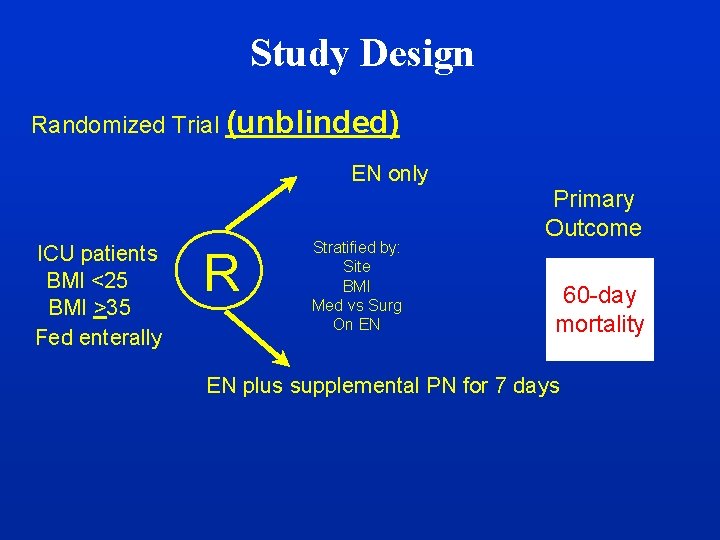
Study Design Randomized Trial (unblinded) EN only ICU patients BMI <25 BMI >35 Fed enterally R Stratified by: Site BMI Med vs Surg On EN Primary Outcome 60 -day mortality EN plus supplemental PN for 7 days

Objectives: Pilot Study Primary Aim: • Difference in the calories and protein received between the control and intervention groups • Estimate recruitment rate • Evaluate the safety, tolerance, and logistics around providing supplemental PN in the study population, e. g. • To ensure adequate glycemic control in both groups • To ensure other metabolic consequences of the feeding strategies are minimized • To establish adequate compliance with study protocols and completion of case report forms. Secondary Aims: • Explore the effect of differential intake of protein/energy on muscle mass and muscle function.

Outcomes: Pilot study Primary outcome: 60 day mortality Secondary outcomes: • • ICU (28 day) mortality Hospital mortality Duration of mechanical ventilation Duration of stay (ICU and hospital) Development of ICU-acquired infections Multiple organ dysfunction (SOFA and PODS) Functional status, HR QOL at 3 & 6 months Muscle Function Tests

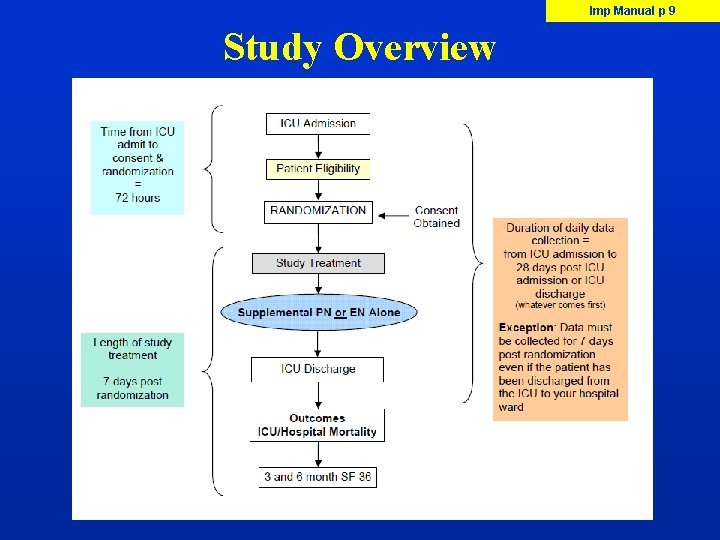
Imp Manual p 9 Study Overview

Pilot Study: Participating Sites Target: 160 patients from 8 institutions Ø Ø Ø Ø Royal Alexandra Hospital, Edmonton (Jim Kutsogiannis) University of Alberta Hospital, Edmonton (Dean Karvellas) University of Colorado, US (Paul Wischmeyer ) Erasme University Hospital, Brussels (Jean Charles Preiser) Hôpitaux Universitaires, Strasbourg, France (Michael Hasselmann) Grey Nun’s Hospital, Edmonton (Dan Stollery) University of Wisconsin (Ken Kudsk) Oregon Health Sciences University (Robert Martindale)

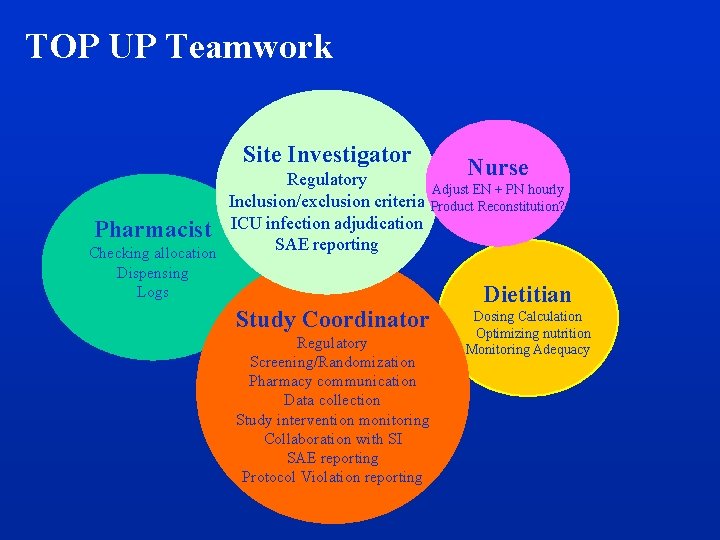
TOP UP Teamwork Site Investigator Regulatory Inclusion/exclusion criteria Pharmacist ICU infection adjudication SAE reporting Checking allocation Dispensing Logs Study Coordinator Regulatory Screening/Randomization Pharmacy communication Data collection Study intervention monitoring Collaboration with SI SAE reporting Protocol Violation reporting Nurse Adjust EN + PN hourly Product Reconstitution? Dietitian Dosing Calculation Optimizing nutrition Monitoring Adequacy

Role of Site Investigator Delegation of Authority Patient Eligibility ICU Infection adjudication SAE identification/assessment Investigator Confirmation

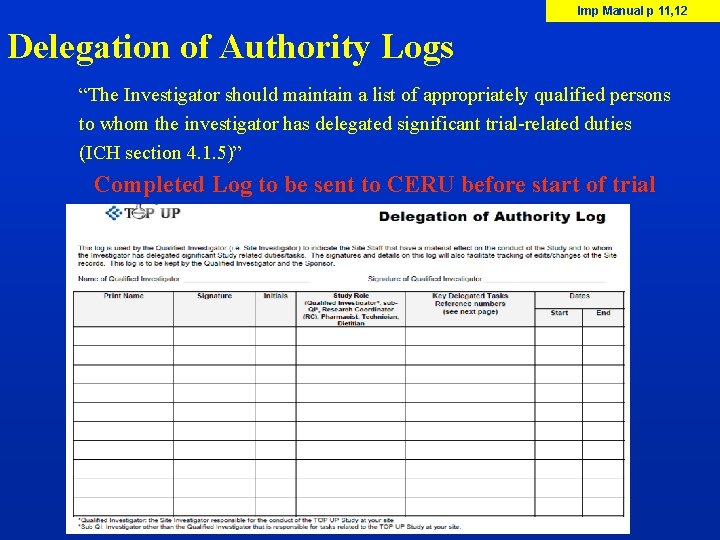
Imp Manual p 11, 12 Delegation of Authority Logs “The Investigator should maintain a list of appropriately qualified persons to whom the investigator has delegated significant trial-related duties (ICH section 4. 1. 5)” Completed Log to be sent to CERU before start of trial

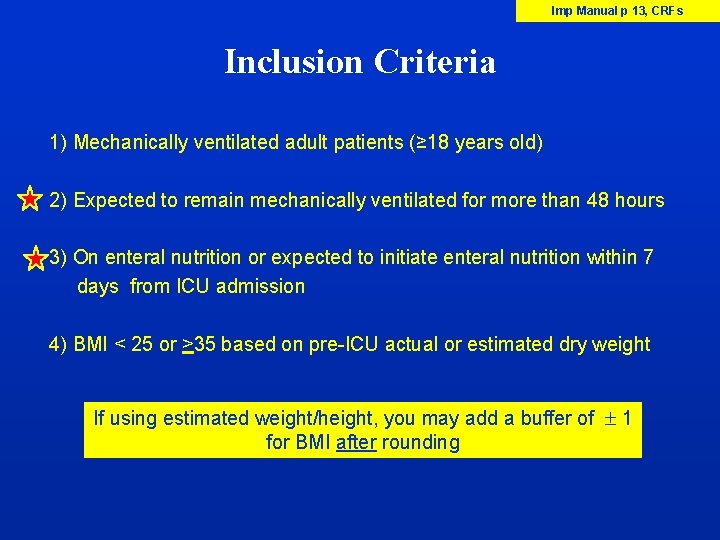
Imp Manual p 13, CRFs Inclusion Criteria 1) Mechanically ventilated adult patients (≥ 18 years old) 2) Expected to remain mechanically ventilated for more than 48 hours 3) On enteral nutrition or expected to initiate enteral nutrition within 7 days from ICU admission 4) BMI < 25 or >35 based on pre-ICU actual or estimated dry weight If using estimated weight/height, you may add a buffer of 1 for BMI after rounding

Imp Manual p 14, CRFs Exclusion Criteria 1. >72 hours from admission to ICU to time of consent (your ICU) 2. Not expected to survive an additional 48 hours from screening evaluation 3. Lack of commitment to full, aggressive care (anticipated withholding or withdrawing treatments in the first week but isolated DNR acceptable) 4. Patients already receiving PN on admission to ICU (does NOT refer to those that received PN in hospital prior to this acute episode of illness)

Imp Manual p 14, CRFs Exclusion Criteria 5. Patients with diabetic ketoacidosis or non ketotic hyperosmolar coma 6. Pregnant or lactating patients 7. Patients with clinical fulminant hepatic failure (see definition) 8. Patients with Cirrhosis Child’s Class C Liver Disease (except those on a transplant list or transplantable) 9. Dedicated port of central line not available 10. Known allergy to study nutrients (soy, egg or olive products) 11. Enrolment in another industry sponsored ICU intervention study (co- enrollment in academic studies will be considered on a case by case basis)

Eligibility confirmation Prompted at time of Pre randomization Refer to Consent Training Slides

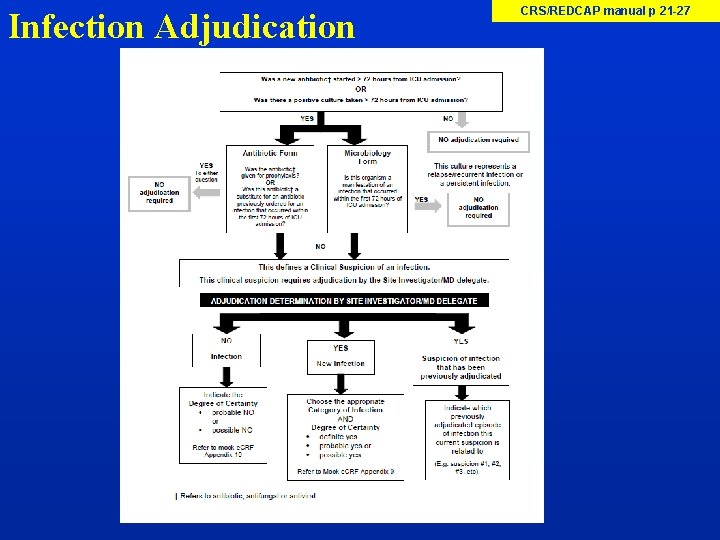
Infection Adjudication Site Investigator to make determination of a newly acquired infection based on antibiotic and microbiology data

Infection Adjudication CRS/REDCAP manual p 21 -27

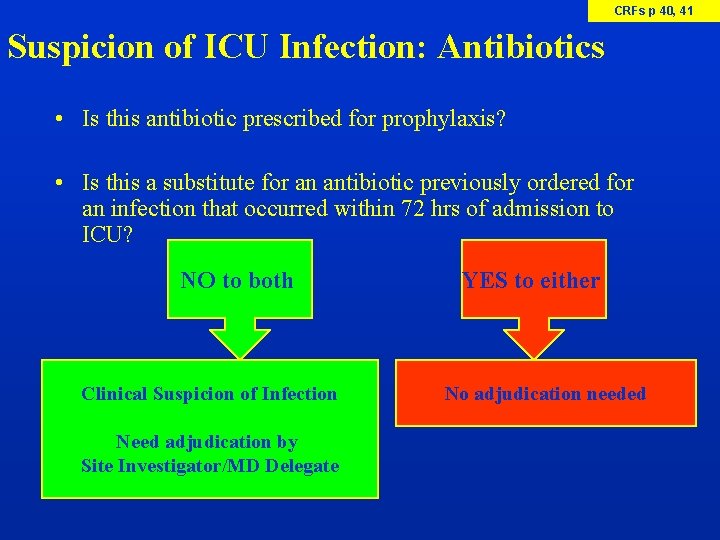
CRFs p 40, 41 Suspicion of ICU Infection: Antibiotics • Is this antibiotic prescribed for prophylaxis? • Is this a substitute for an antibiotic previously ordered for an infection that occurred within 72 hrs of admission to ICU? NO to both Clinical Suspicion of Infection Need adjudication by Site Investigator/MD Delegate YES to either No adjudication needed

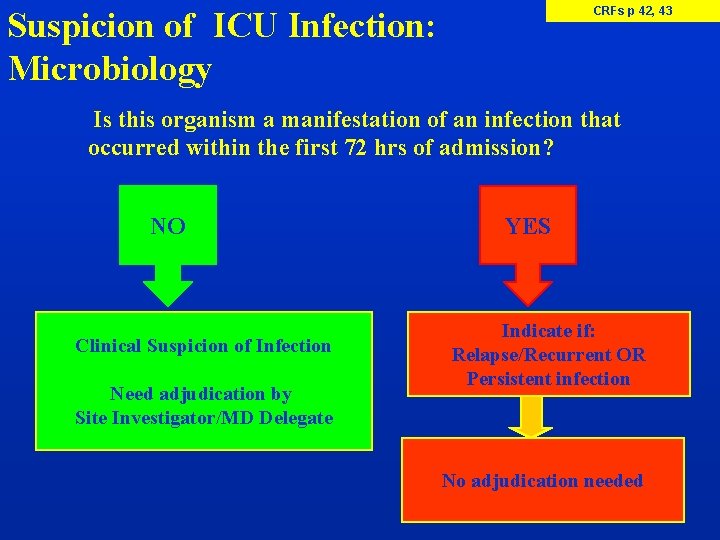
CRFs p 42, 43 Suspicion of ICU Infection: Microbiology Is this organism a manifestation of an infection that occurred within the first 72 hrs of admission? NO Clinical Suspicion of Infection Need adjudication by Site Investigator/MD Delegate YES Indicate if: Relapse/Recurrent OR Persistent infection No adjudication needed

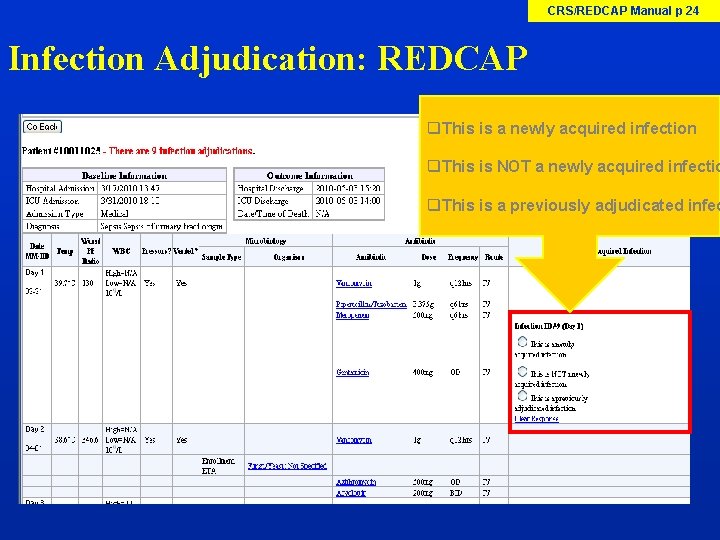
CRS/REDCAP Manual p 24 Infection Adjudication: REDCAP q. This is a newly acquired infection q. This is NOT a newly acquired infectio q. This is a previously adjudicated infec

Infection Adjudication Site Investigator will need: 1. Access to view the Infection Adjudication table on REDCAP (Research Coordinator to show this) 2. Appendix 9 Categories of Infection 3. Appendix 10 Definitions of No Newly Acquired Infection 4. Medical Chart Refer to CRS/REDCAP Manual pages 21 -27 for step by step process

SAE Identification and Reporting

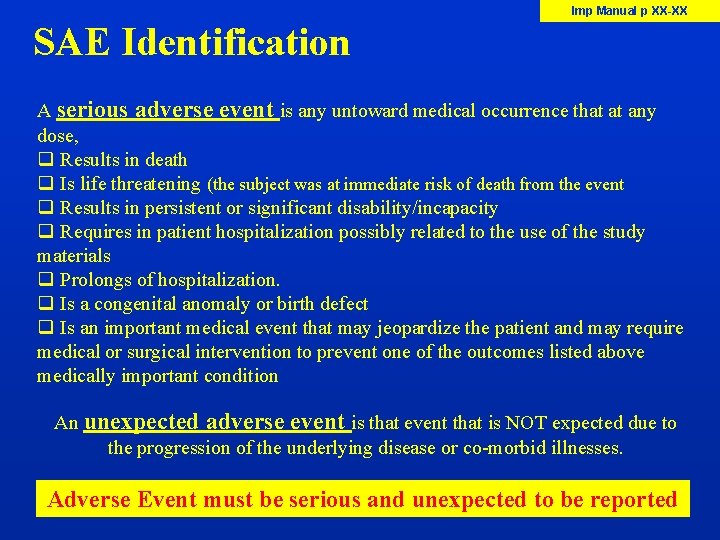
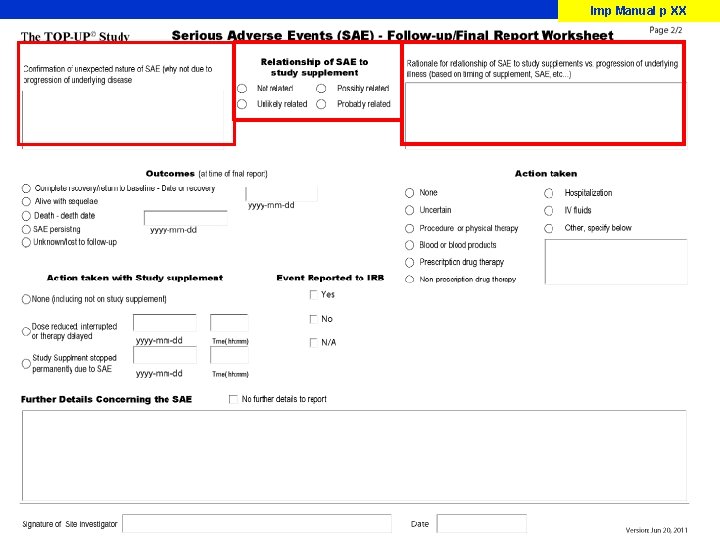
Imp Manual p XX-XX SAE Identification A serious adverse event is any untoward medical occurrence that at any dose, q Results in death q Is life threatening (the subject was at immediate risk of death from the event q Results in persistent or significant disability/incapacity q Requires in patient hospitalization possibly related to the use of the study materials q Prolongs of hospitalization. q Is a congenital anomaly or birth defect q Is an important medical event that may jeopardize the patient and may require medical or surgical intervention to prevent one of the outcomes listed above medically important condition An unexpected adverse event is that event that is NOT expected due to the progression of the underlying disease or co-morbid illnesses. Adverse Event must be serious and unexpected to be reported

SAE Reporting (to CERU) Imp Manual p X Must be done on electronic data capture system and faxed to CERU

Imp Manual p XX-XX

Imp Manual p XX-XX

Imp Manual p XX

Imp Manual appendix J

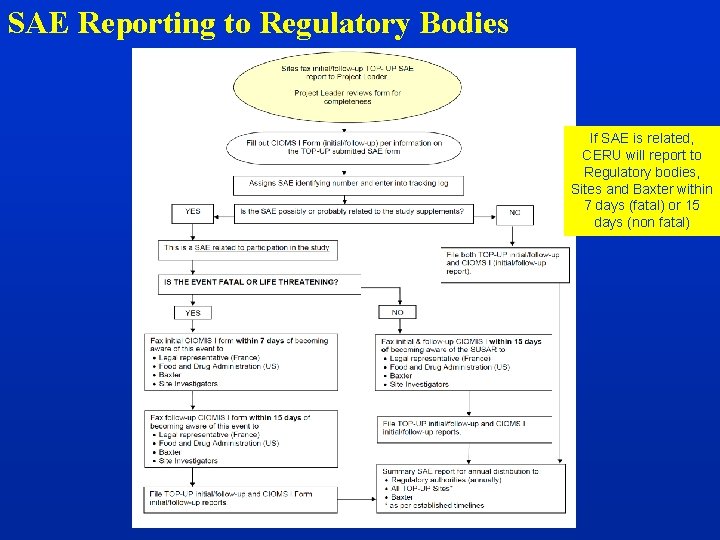
SAE Reporting to Regulatory Bodies If SAE is related, CERU will report to Regulatory bodies, Sites and Baxter within 7 days (fatal) or 15 days (non fatal)

CRS/REDCAP Manual p 30 Investigator Confirmation

Imp Manual p X Study Groups Name of Group Intervention Supplemental PN EN (enteral nutrition) plus Olimel EN only EN

Dosing Procedures (both groups) Dosing of the intervention will depend upon the energy and protein needs of the patient To be determined by the dietitian/MD

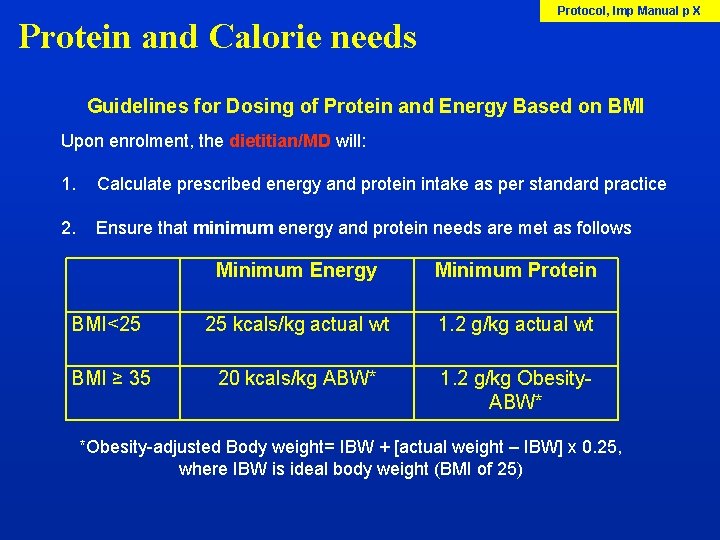
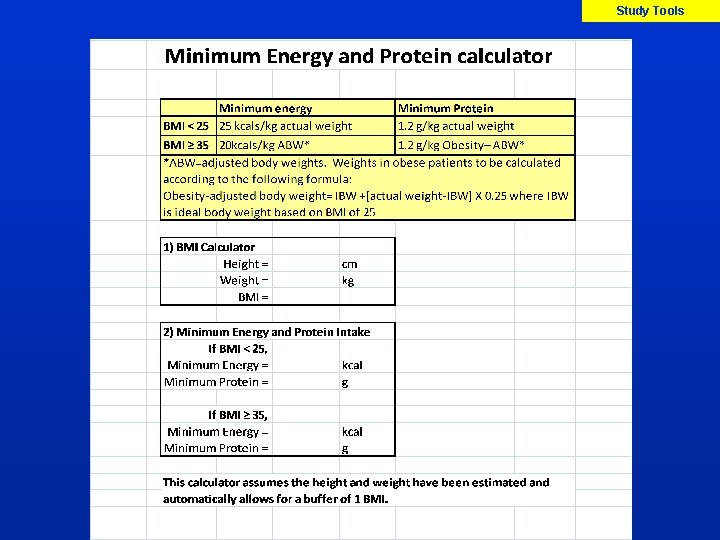
Protein and Calorie needs Protocol, Imp Manual p X Guidelines for Dosing of Protein and Energy Based on BMI Upon enrolment, the dietitian/MD will: 1. Calculate prescribed energy and protein intake as per standard practice 2. Ensure that minimum energy and protein needs are met as follows Minimum Energy Minimum Protein BMI<25 25 kcals/kg actual wt 1. 2 g/kg actual wt BMI ≥ 35 20 kcals/kg ABW* 1. 2 g/kg Obesity. ABW* *Obesity-adjusted Body weight= IBW + [actual weight – IBW] x 0. 25, where IBW is ideal body weight (BMI of 25)

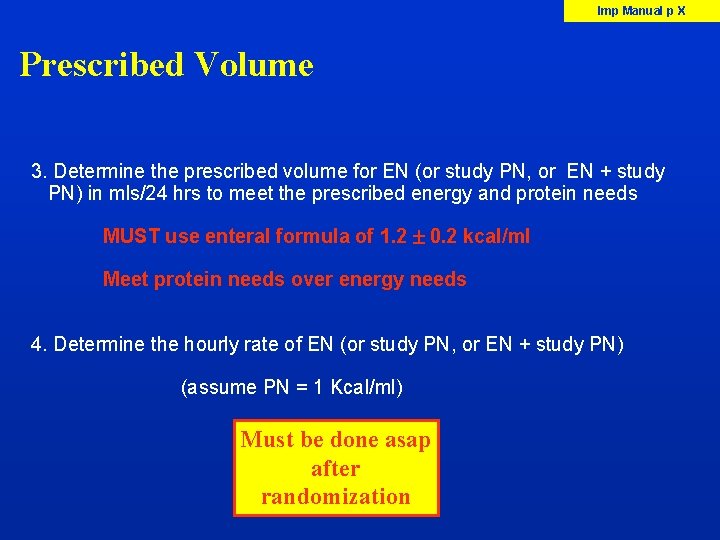
Imp Manual p X Prescribed Volume 3. Determine the prescribed volume for EN (or study PN, or EN + study PN) in mls/24 hrs to meet the prescribed energy and protein needs MUST use enteral formula of 1. 2 0. 2 kcal/ml Meet protein needs over energy needs 4. Determine the hourly rate of EN (or study PN, or EN + study PN) (assume PN = 1 Kcal/ml) Must be done asap after randomization

Study Tools

Other considerations…Propofol calories to be factored into assessment of caloric needs, only as per discretion of dietitian/MD

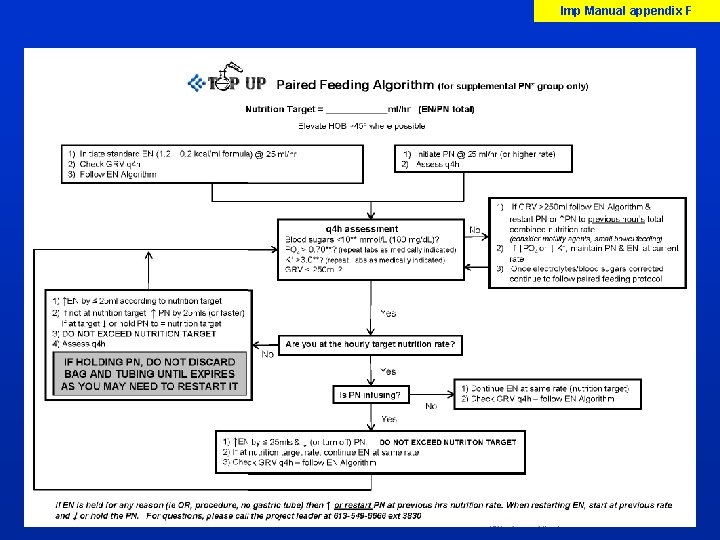
Imp Manual p X Enteral Nutrition (both groups) • Enteral Nutrition to start as per usual practice (patient stabilized, NG/Feeding tube in place) • Standard enteral nutrition formula 1. 0 to 1. 4 kcals/ml » (hypercaloric formulas not allowed) » NO protein supplements (for 7 days) » NO probiotics (for 7 days) » NO glutamine supplements (for 7 days) • Start at 25 ml/hr and increase every 4 hrs as tolerated until goal rate • Discontinue when the feeding tube comes out Refer to Enteral Nutrition Algorithm & Paired Feeding Algorithm appendices C & F

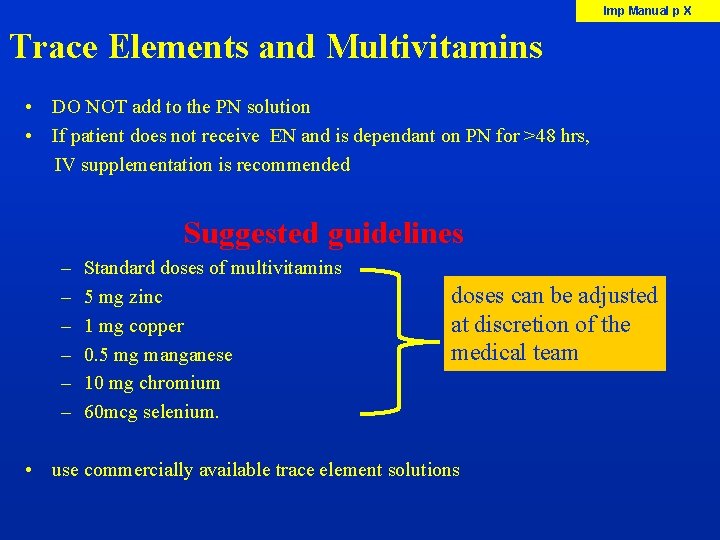
Imp Manual p X Trace Elements and Multivitamins • DO NOT add to the PN solution • If patient does not receive EN and is dependant on PN for >48 hrs, IV supplementation is recommended Suggested guidelines – – – Standard doses of multivitamins 5 mg zinc 1 mg copper 0. 5 mg manganese 10 mg chromium 60 mcg selenium. doses can be adjusted at discretion of the medical team • use commercially available trace element solutions

Dietitian Determine Energy/protein needs (prescribed Volume) Follow Canadian Clinical Practice Guidelines Assist with data collection Baseline Nutrition Assessment Daily EN monitoring Daily PN monitoring (non study PN)

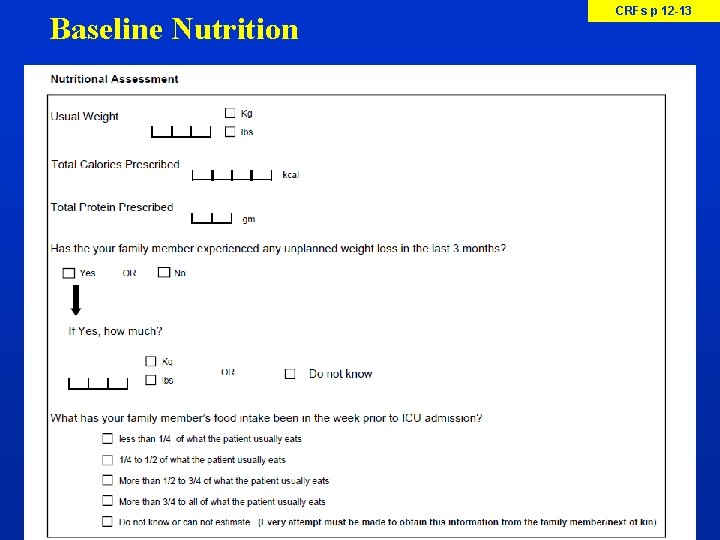
Baseline Nutrition CRFs p 12 -13

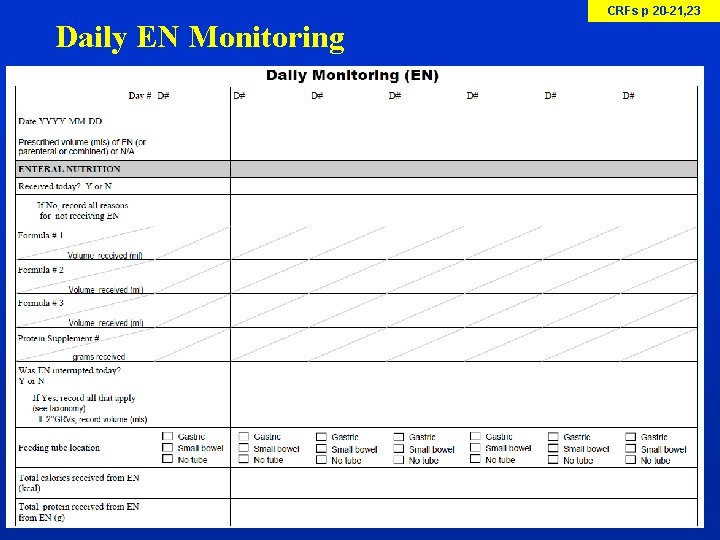
CRFs p 20 -21, 23 Daily EN Monitoring

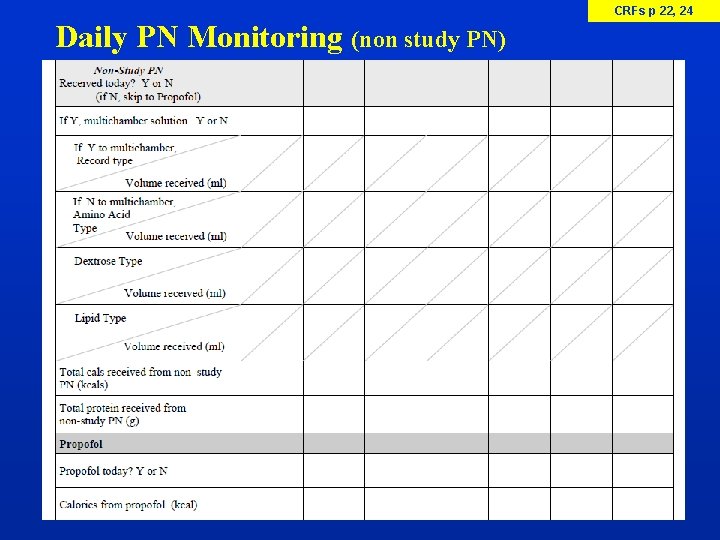
CRFs p 22, 24 Daily PN Monitoring (non study PN)

CRFs p 3 Study Days and Data Collection Data MUST be collected according to calendar day as described below Do NOT collect data according to your flow sheet unless it runs from 00: 00 to 23: 59 (midnight to midnight)

Supplemental PN group

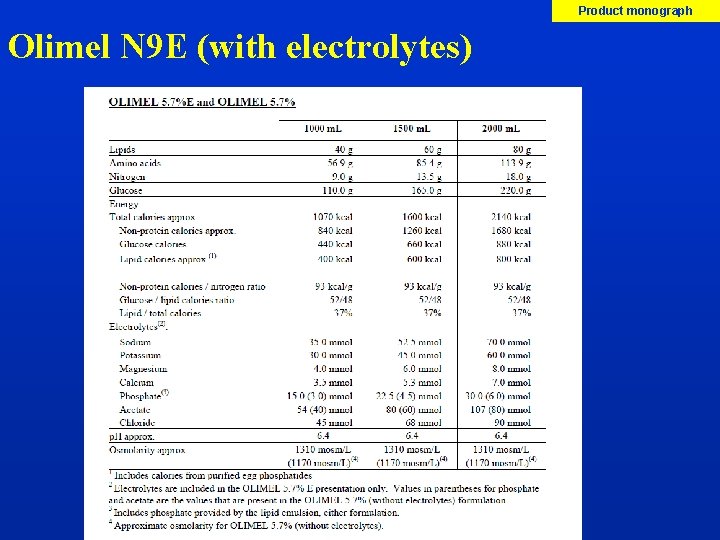
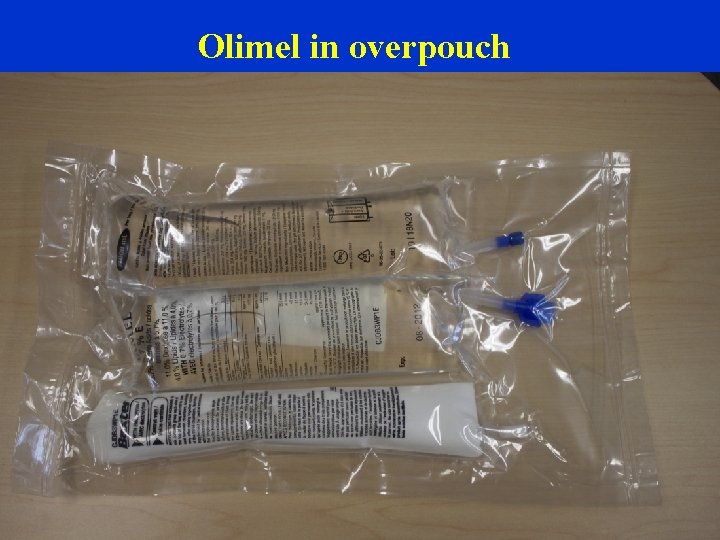
Imp Manual p XX, XX Olimel N 9 E (5. 7%) Amino acids 3 -in-1 PN solution that contains glucose, lipid emulsion and an amino acids Dextrose Blend of desirable lipids: olive oil, soybean oil (ratio 80/20) Alpha-tocopherol/moderate PUFA, better vitamin E status less lipid peroxidation. Protein and energy content of Olimel N 9 E enables the maintenance of an adequate nitrogen/energy balance Lipid emulsion Spike insertion site 1 Litre bags, provided by Baxter

Product monograph Olimel N 9 E (with electrolytes)

Imp Manual p X When to start Olimel? Start as soon as central line access Preferably within 2 hrs of randomization

Imp Manual p XX-XX Paired Feeding § Enteral and parenteral solutions provided continuously over 24 h § Rate of PN depends upon rate of EN § Adjust PN hourly so that EN + PN = target rate as determined by dietitian/MD § Initiate PN study solution at 25 ml/hr (or faster) and advance by 25 ml q 4 hrs to target rate as tolerated § Monitor blood sugars and electrolytes every 4 hrs as needed § Do not advance PN/EN if BS, K, Phosp, Mag becoming more abnormal (ranges as per your local site) To reach the target combined rate by EN plus PN within 24 hrs from randomization

Imp Manual appendix F

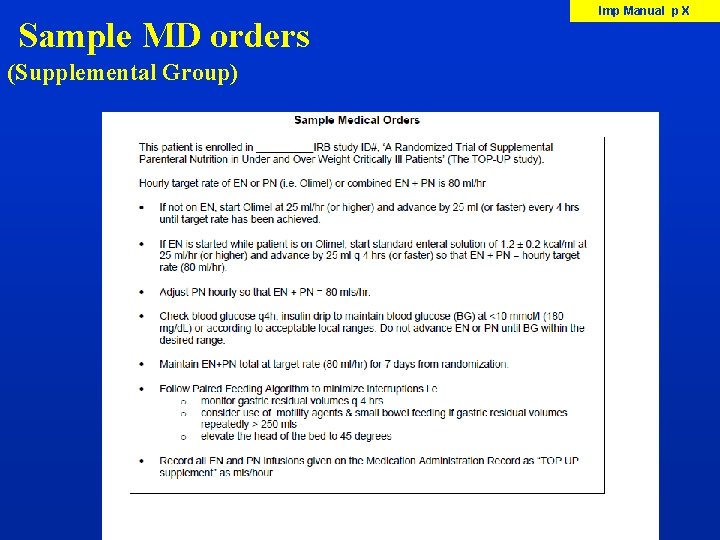
Sample MD orders (Supplemental Group) Imp Manual p X

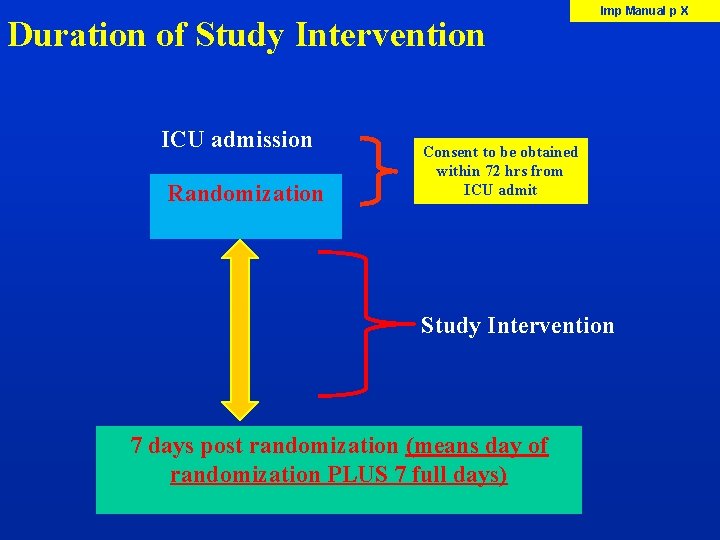
Duration of Study Intervention ICU admission Randomization Imp Manual p X Consent to be obtained within 72 hrs from ICU admit Study Intervention 7 days post randomization (means day of randomization PLUS 7 full days)

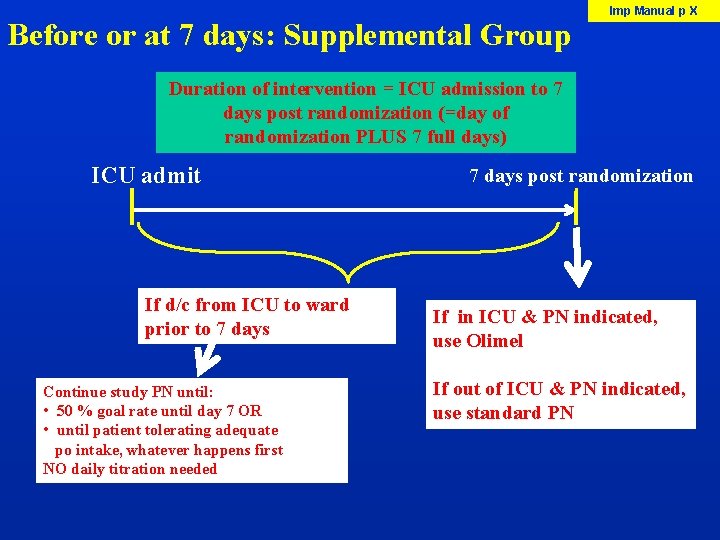
Before or at 7 days: Supplemental Group Imp Manual p X Duration of intervention = ICU admission to 7 days post randomization (=day of randomization PLUS 7 full days) ICU admit If d/c from ICU to ward prior to 7 days Continue study PN until: • 50 % goal rate until day 7 OR • until patient tolerating adequate po intake, whatever happens first NO daily titration needed 7 days post randomization If in ICU & PN indicated, use Olimel If out of ICU & PN indicated, use standard PN

Imp Manual p X Use of Non Study Parenteral Nutrition If parenteral nutrition is truly indicated § In ICU: use Olimel until study day 28 maximum § When on floor after ICU: use standard PN Both groups: If non study PN received before 7 days PROTOCOL VIOLATION Report to CERU asap

EN only group

Imp Manual p X Enteral Nutrition • Enteral Nutrition to start as per usual practice (patient stabilized and NG/Feeding tube in place) • Standard enteral nutrition formula • 1. 0 to 1. 4 kcals/ml » (hypercaloric formulas not allowed) » NO protein supplements (for 7 days) » NO probiotics (for 7 days) » NO glutamine supplements (for 7 days) • Start at 25 ml/hr (or and increased every 4 hrs as tolerated until goal rate • Discontinue when the feeding tube comes out Refer to Enteral Nutrition Algorithm

Imp Manual appendix C

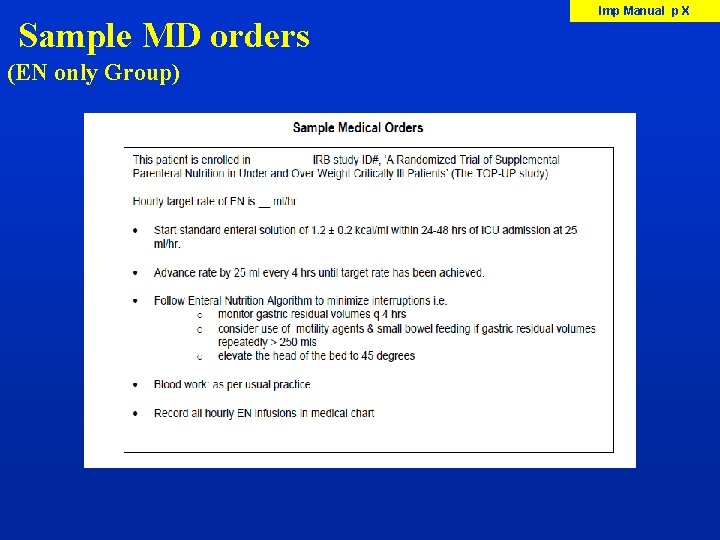
Sample MD orders (EN only Group) Imp Manual p X

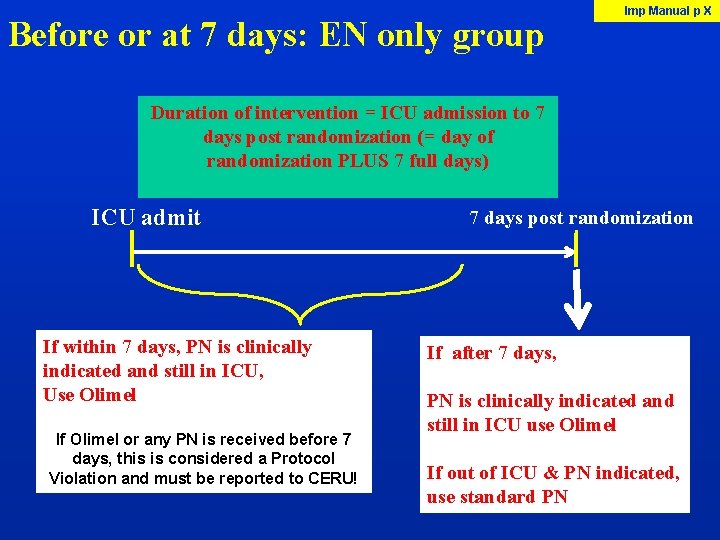
Before or at 7 days: EN only group Imp Manual p X Duration of intervention = ICU admission to 7 days post randomization (= day of randomization PLUS 7 full days) ICU admit If within 7 days, PN is clinically indicated and still in ICU, Use Olimel If Olimel or any PN is received before 7 days, this is considered a Protocol Violation and must be reported to CERU! 7 days post randomization If after 7 days, PN is clinically indicated and still in ICU use Olimel If out of ICU & PN indicated, use standard PN

Both groups EN only & Supplemental PN group

Imp Manual p X Trace Elements and Multivitamins • DO NOT add to the PN solution • If patient does not receive EN and is dependant on PN for >48 hrs, IV supplementation is recommended Suggested guidelines – – – Standard doses of multivitamins 5 mg zinc 1 mg copper 0. 5 mg manganese 10 mg chromium 60 mcg selenium. doses can be adjusted at discretion of the medical team • use commercially available trace element solutions

Imp Manual p X Co-interventions Follow Canadian Nutrition Guidelines Glycemic Control Protocol Daily sedation vacations Sepsis management guidelines Daily trials of weaning from mechanical ventilation

Investigational Product Dispensing & Storage

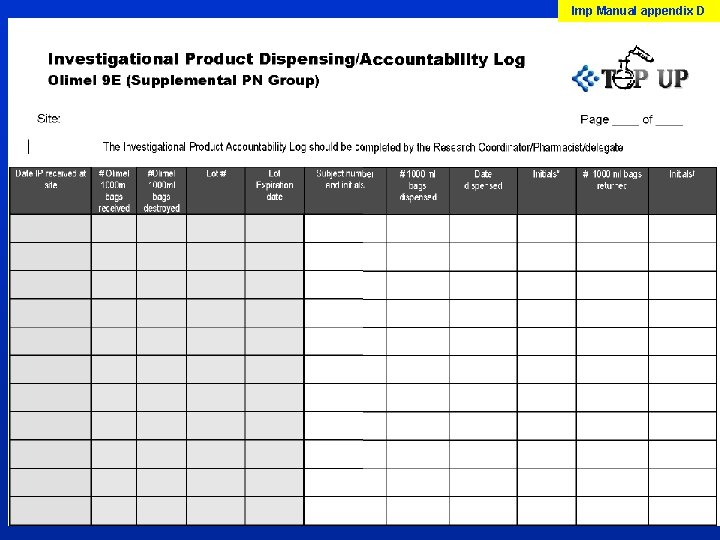
Pharmacy For most sites, the pharmacy may only be involved in the initial receipt of the Olimel. The Research Coordinator/delegate will therefore be responsible for the following: q storage of Olimel q dispensing of Olimel (including addition of labels) q completion of dispensing and accountability logs q sending temp logs to CERU monthly q maintenance of inventory and destruction of the expired/unused product

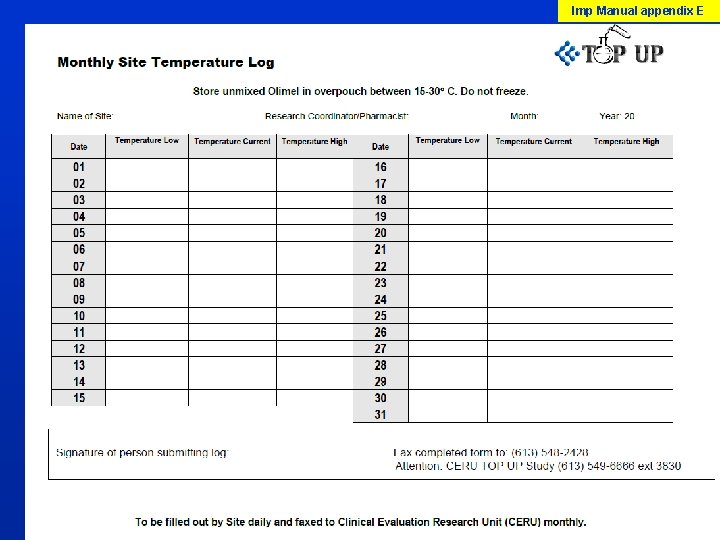
Imp Manual p X Olimel N 9 E Will be supplied to all sites before enrollment starts At time of delivery, if pre-activation occurs (solution has turned milky) do not use and report to CERU Project Leader Unmixed product: store between 15 to 30 degrees C. Do not freeze. Dedicated Central line needed, piggybacking with other lines not recommended unless standard practice for PN at your site

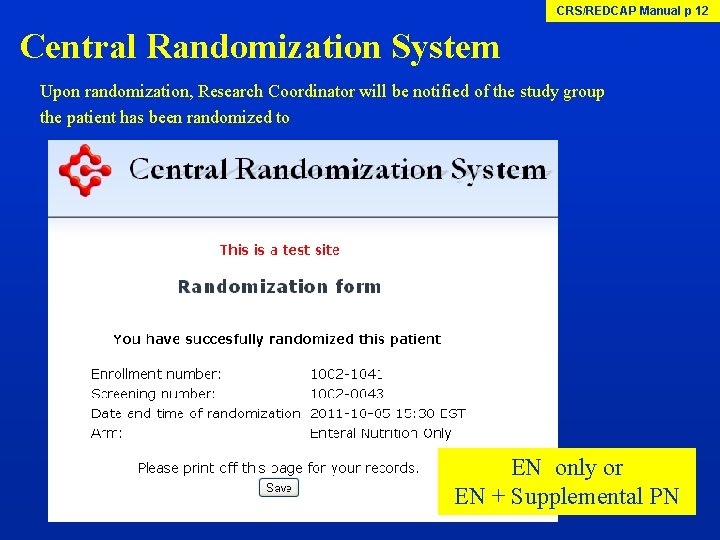
CRS/REDCAP Manual p 12 Central Randomization System Upon randomization, Research Coordinator will be notified of the study group the patient has been randomized to EN only or EN + Supplemental PN

Imp Manual p X Supplemental PN For day 1: Research Coordinator to obtain hourly rate of infusion from dietitian/MD Prepare enough 1 litre bags of the investigational product to last one day Example: Dietitian/MD has determined that the hourly rate is 65 ml/hr: the total volume needed for 1 day would be 65 X 24 = 1536 mls. The pharmacist /delegate is to prepare 2 X 1 Litre bags of the product. In order to prevent running out of product before the bag change time, you may need to send 2 X 1 litre bags on day 1

Supplemental PN For Subsequent days: The Research Coordinator to determine how much enteral nutrition the patient is anticipated to tolerate and will prepare enough Olimel accordingly. Example: 1. Goal rate = 65 ml/hr 2. Patient tolerating 25 ml/hr well today (or expected to) 3. Prepare enough product for remaining volume = 40 ml/hr X 24 = 960 mls = 1 X 1 litre bag

Olimel in overpouch

Imp Manual p X Labels Study: The TOP-UP Study ID #: NCT 01206166 Olimel N 9 E PARENTERAL USE ONLY Canadian Sponsor: Dr. Daren Heyland Clinical Evaluation Research Unit, Kingston General Hospital, 76 Stuart St, Kingston, ON K 7 L 2 V 7 Randomization #: _____ Patient ID: ________ Patient Name: _______ Directions: Run at maximum goal rate of XX ml/hr and titrate down as enteral feeds increase. Storage: Room temperature Expiration: 24 hrs Unblinded labels Appx 3” x 5” size Generate and attach 1 label for outside of reconstituted bag Labels must be placed on extra bags needed for after hours (expiration date and time must be recorded…. . by RN)

Imp Manual appendix D

Imp Manual appendix E

Imp Manual p X Nursing Procedures Training Slides available on Reconstitution of Olimel www. criticalcare nutrition. com

Reconstitution of Olimel done by Nurse and/or Research Coordinator

Olimel in overpouch

After removing pouch, check the Oxygen indicator Black Light yellow brown After overwrap has been removed, Olimel can be stored for 24 hours under refrigeration followed by 24 hours administration

Check non permanent seals Confirm the integrity of the bag and of the non-permanent seals Use only if the bag is ü not damaged, if the non-permanent seals are intact (i. e. no mixture of the contents of the three compartments) ü if the amino acids solution and the glucose solution are clear, colourless practically free of visible particles, and ü if the lipid emulsion is a homogeneous liquid with a milky appearance

1. Ensure that the product is at room temperature when breaking the non- permanent seals 2. After removing overpouch, manually roll bag from hanger side down

Continue rolling the bag until all non permanent seals are broken ½ way

Solution turns milky showing that the seals are broken (1/2 way)


Mix well by inverting the bag 3 times

1. Solution will turn to a milky color & is ready to hang 2. Use immediately after reconstitution

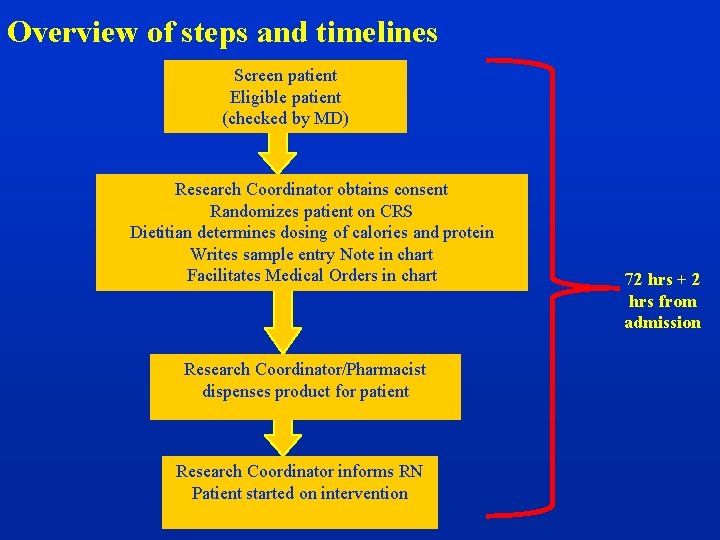
Overview of steps and timelines Screen patient Eligible patient (checked by MD) Research Coordinator obtains consent Randomizes patient on CRS Dietitian determines dosing of calories and protein Writes sample entry Note in chart Facilitates Medical Orders in chart Research Coordinator/Pharmacist dispenses product for patient Research Coordinator informs RN Patient started on intervention 72 hrs + 2 hrs from admission

Muscle Function Tests

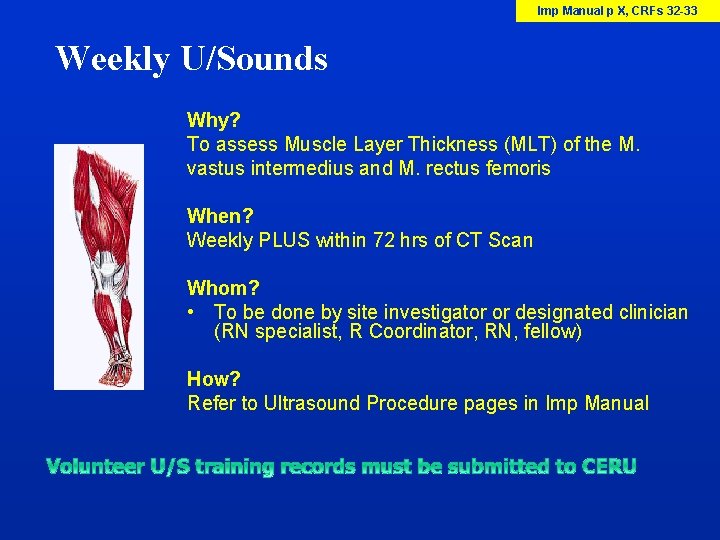
Imp Manual p X, CRFs 32 -33 Weekly U/Sounds Why? To assess Muscle Layer Thickness (MLT) of the M. vastus intermedius and M. rectus femoris When? Weekly PLUS within 72 hrs of CT Scan Whom? • To be done by site investigator or designated clinician (RN specialist, R Coordinator, RN, fellow) How? Refer to Ultrasound Procedure pages in Imp Manual

CRF p 34 -35 Abdominal/Pelvic CT Scan Why? Assess muscle mass (at 3 rd lumbar vertebrae) as a predictor of lean tissue mass When? CT Scans done 1 -2 days prior or after ICU admission and all subsequent scans Whom? Research Coordinator to retrieve scans of previously done CTs and obtain copies and send DE-IDENTIFIED to University of Waterloo How? • CT Images already performed for clinical reasons • Not to be done for the study if not clinically indicated Mourtzakis M et al Critical Care Canada Forum, 2009.

Imp Manual p X Hand Grip Strength Why? To assess the physical strength When? ICU and hospital discharge Whom? To be done by the Research Coordinator How? • On a patient that is awake and attentive, upright with elbow at 90 degrees • Using a hand dynamometer (Jamar) on dominant hand, three readings (sustained 5 sec, rest for 15 sec between) • REFER TO HAND GRIP STRENGTH TEST MODULE (SLIDES)

Imp Manual p X 6 Minute Walk Test Why? One time measure of functional status of patients When? Prior to hospital discharge Whom? To be done by the Research Coordinator How? • • • Calculate the total distance walked by patient in 6 minutes. On a patient that is able to walk, need a long corridor (30 metres) Patients with recent unstable angina are excluded Ensure that the patient is safe, chair nearby Worksheets, specific instructions, script provided

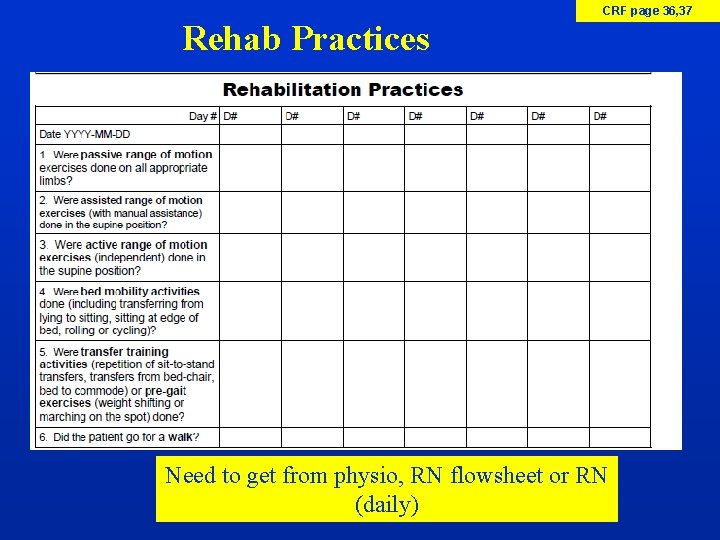
CRF page 36, 37 Rehab Practices Need to get from physio, RN flowsheet or RN (daily)

Imp Manual Research Coordinator Procedures Consent (Training Module) Training of nurses Data Collection Protocol Violations

CRFs April 12 th 2012 Case Report Forms

Duration of Data Collection

The duration of daily data collection and frequency will vary depending upon each data element/form and is as follows : · Collected once: Baseline Barthel ADL Index, Baseline SF-36, Nutritional Assessment, Baseline, Nutrition Timing, Ventilation/Dialysis, Outcomes Barthel ADL Index, Muscle Function Testing (6 -min Walk Test & Hand Grip Strength Test only), Hospitalization Overview, 3 -month SF-36 Follow-up and 6 -month SF-36 Follow-up · Daily from Study Day 1 until ICU discharge or death for a maximum of 28 days from ICU admission: Daily Nutrition Monitoring, Daily Organ Dysfunction, Daily Laboratory and Intra Abdominal Pressure, Rehabilitation Practices and Concomitant Medications · Daily from Study Day 1 until 3 days after ICU discharge or death for a maximum of 28 days: Antibiotic/Antiviral/Antifungal and Microbiology · Weekly/Other specified intervals: Muscle Function Testing (Weekly study Femoral Ultrasounds) and Abdominal/Pelvis CT Scans/Femoral Ultrasounds

Duration of Study Intervention

The duration of the study intervention is: 7 days post randomization* or until death whichever comes first Exceptions: If the patient is discharged from the ICU to your hospital ward before 7 days: • Supplemental PN Group: Continue PN intervention until day 7 post randomization* or until the patient is tolerating adequate amounts of oral nutrition (i. e. > 50% caloric goal orally) • Both groups: Collect daily data from Study Day 1 until 7 days post randomization* • Both groups: Collect antibiotic and microbiology data from Study Day 1 until 10 days post randomization** *7 Days post randomization = Day of randomization Plus 7 full days **10 Days post randomization = Day of randomization Plus 10 full days EXAMPLE: Study Day 1 = Patient admitted to ICU Aug 30 @ 23: 20 Study Day 2 = Patient randomized to TOP-UP Aug 31 @ 12: 35 Study Day 8 = Patient discharged from ICU Sept 6 @ 18: 04 Study Day 9 = Last day of Daily Data Collection (7 days post randomization*) Sep 7 @ 23: 59 Study Day 12 = Last day of Antibiotic and Microbiology collection (10 days post randomization**) Sep 10 @ 23: 59

CRS/REDCAP Manual April 11 th 2012

Resources online www. criticalcarenutrition. com

 Advantage of randomized controlled trial
Advantage of randomized controlled trial Small bowel obstruction parenteral nutrition
Small bowel obstruction parenteral nutrition Calculating tpn calories
Calculating tpn calories Enteral
Enteral Continuous parenteral nutrition
Continuous parenteral nutrition Tpn complication
Tpn complication Complication of parenteral nutrition
Complication of parenteral nutrition Total parenteral nutrition cost
Total parenteral nutrition cost Ppn vs tpn
Ppn vs tpn Randomized algorithms and probabilistic analysis
Randomized algorithms and probabilistic analysis Randomized algorithm in daa
Randomized algorithm in daa Matched pairs design
Matched pairs design Switching replications design
Switching replications design Rcbd meaning in research
Rcbd meaning in research Complete block design
Complete block design Randomized polynomial time
Randomized polynomial time Randomized polynomial time
Randomized polynomial time Two-way anova table
Two-way anova table Expected running time
Expected running time Duncan's multiple range test
Duncan's multiple range test Statistical model for crd
Statistical model for crd Complete randomized design example
Complete randomized design example Pronounce nivolumab
Pronounce nivolumab Completely randomized design
Completely randomized design Completely randomized design
Completely randomized design Rbd experimental design
Rbd experimental design Crd rcbd
Crd rcbd Factorial randomized block design
Factorial randomized block design Randomized hill climbing
Randomized hill climbing Randomized skip list
Randomized skip list Randomized hill climbing
Randomized hill climbing Types of randomized algorithms
Types of randomized algorithms What is supplemental security income
What is supplemental security income School of nursing ubc
School of nursing ubc Science staar supplemental aids
Science staar supplemental aids Utsa si
Utsa si Supplemental instruction
Supplemental instruction Mcas accommodations manual 2020
Mcas accommodations manual 2020 Supplemental figure
Supplemental figure Supplemental figures
Supplemental figures Galliform with supplemental molt
Galliform with supplemental molt Tea approved graphic organizers
Tea approved graphic organizers Supplemental logging
Supplemental logging Iowa state vet school supplemental application
Iowa state vet school supplemental application What is ssi
What is ssi Supplemental table
Supplemental table What is supplemental security income
What is supplemental security income Supplemental table
Supplemental table Supplementary figure 1
Supplementary figure 1 Staar multiplication chart
Staar multiplication chart Supplemental instruction
Supplemental instruction Mark covin ucla
Mark covin ucla Alter table add supplemental log data all columns
Alter table add supplemental log data all columns What is menu evaluation
What is menu evaluation Supplemental instruction
Supplemental instruction Supplemental table 1
Supplemental table 1 Supplemental online coursework
Supplemental online coursework Large volume parentral
Large volume parentral Dosage calculations formulas
Dosage calculations formulas Dieta enteral e parenteral
Dieta enteral e parenteral Osmolaridad de nutricion parenteral
Osmolaridad de nutricion parenteral Contoh sediaan parenteral volume kecil
Contoh sediaan parenteral volume kecil Difference between oral and parenteral route
Difference between oral and parenteral route Betalatamicos
Betalatamicos Outpatient parenteral antimicrobial therapy (opat)
Outpatient parenteral antimicrobial therapy (opat) Parenteral beslenme komplikasyonları
Parenteral beslenme komplikasyonları Parenteral beslenme komplikasyonları
Parenteral beslenme komplikasyonları Vía intramuscular
Vía intramuscular Hipotonis
Hipotonis Dieta enteral e parenteral
Dieta enteral e parenteral Plan hidratacion oms
Plan hidratacion oms Dermoject
Dermoject Classification of dosage forms
Classification of dosage forms Aislamiento inverso hospitalario
Aislamiento inverso hospitalario Enteral beslenmenin komplikasyonları
Enteral beslenmenin komplikasyonları Chart of dosage form
Chart of dosage form Apendicitis
Apendicitis Penrose drain
Penrose drain Topical routes
Topical routes Aminovel
Aminovel Iv admixture program
Iv admixture program Obat parental
Obat parental Continuous feeding vs bolus feeding
Continuous feeding vs bolus feeding Starter tpn
Starter tpn Ostoma
Ostoma Nonparenteral
Nonparenteral Parenteral dosage calculations
Parenteral dosage calculations Parenteral emulsion
Parenteral emulsion Left ventrogluteal
Left ventrogluteal Enteral parenteral beslenme
Enteral parenteral beslenme Parenteral calculations
Parenteral calculations Nutrisi enteral dan parenteral
Nutrisi enteral dan parenteral Quality control test for parenteral
Quality control test for parenteral Pharmacological and parenteral therapies
Pharmacological and parenteral therapies Gastrostomia
Gastrostomia Iwr ivr clinical
Iwr ivr clinical Lotus domino server
Lotus domino server Trialschule elmar
Trialschule elmar Street law mock trial
Street law mock trial Phitt
Phitt Licencje co to
Licencje co to Chris archer mock trial
Chris archer mock trial Drafft
Drafft Tracheostomy downsizing protocol
Tracheostomy downsizing protocol Subjective probability example
Subjective probability example