A Randomized Phase III Trial of the Value
















- Slides: 51

A Randomized Phase III Trial of the Value of Early Local Therapy for the Intact Primary Tumor in Patients with Metastatic Breast Cancer: ECOG-ACRIN 2108 Presented By Seema Khan at TBD

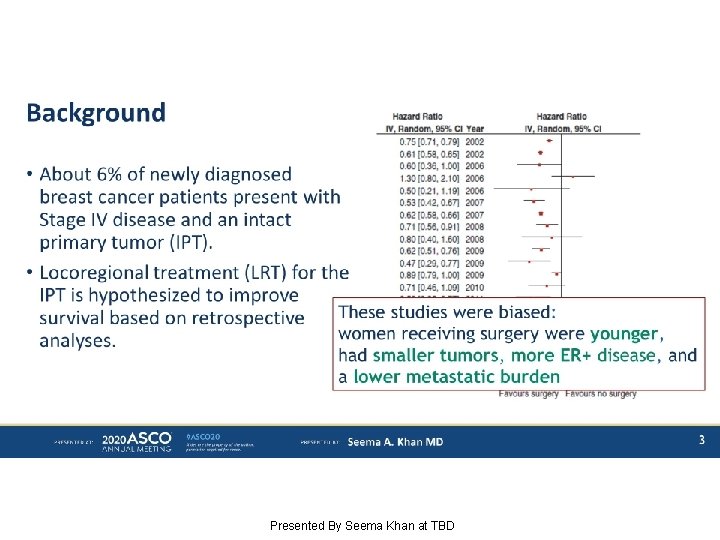
Background Presented By Seema Khan at TBD

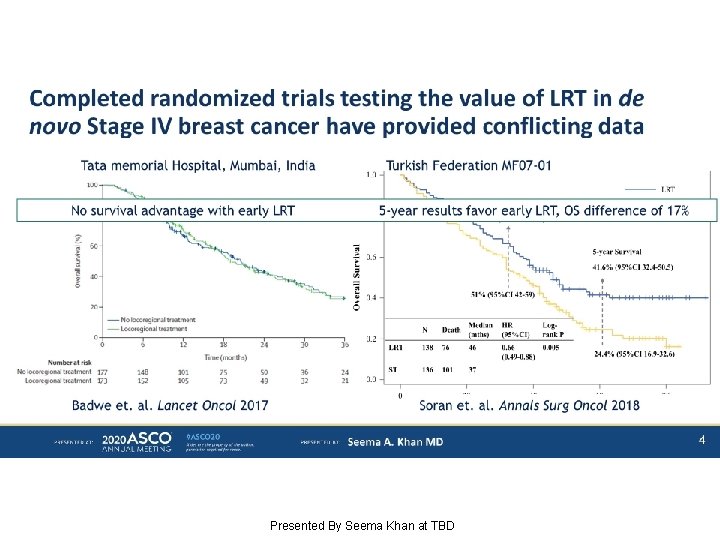
Completed randomized trials testing the value of LRT in de novo Stage IV breast cancer have provided conflicting data Presented By Seema Khan at TBD

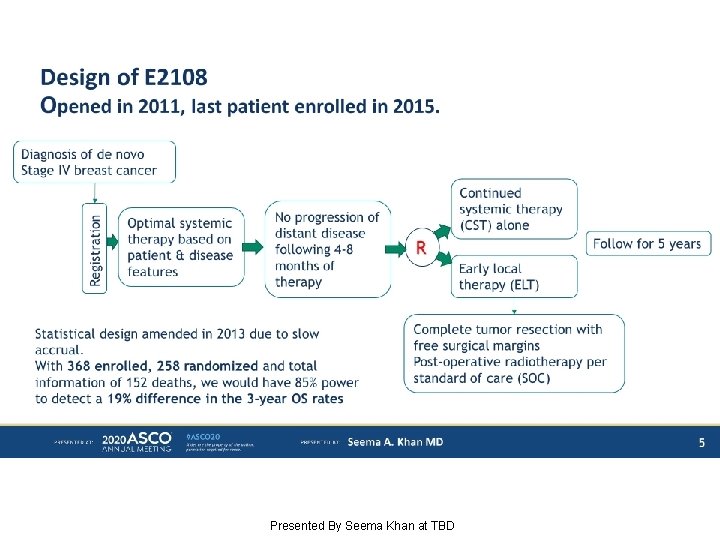
Design of E 2108 Opened in 2011, last patient enrolled in 2015. Presented By Seema Khan at TBD

Endpoints Presented By Seema Khan at TBD

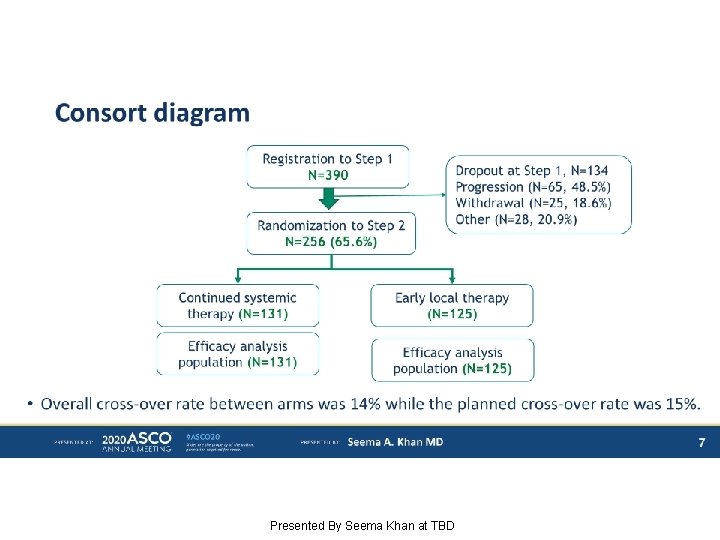
Consort diagram Presented By Seema Khan at TBD

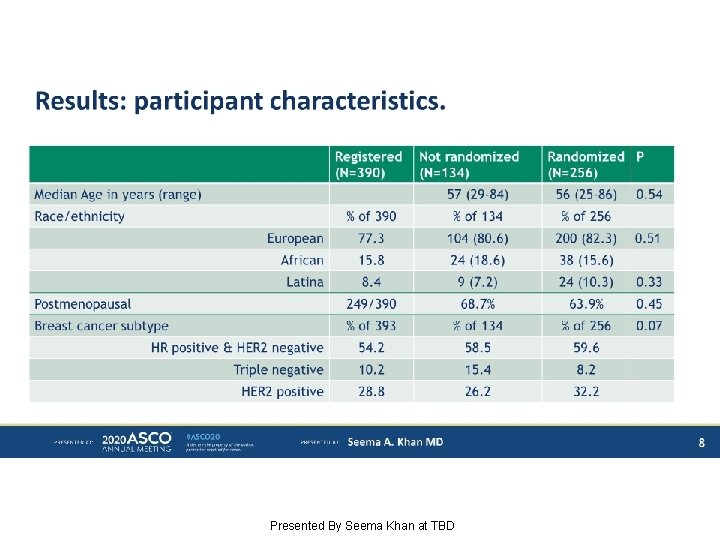
Results: participant characteristics. Presented By Seema Khan at TBD

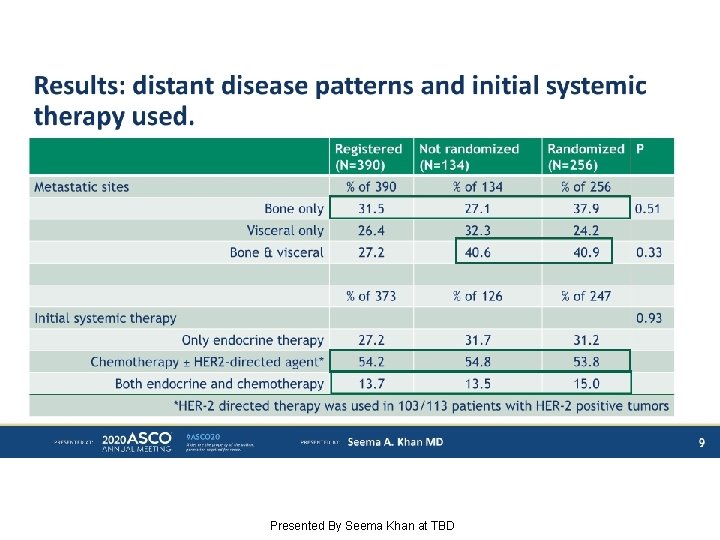
Results: distant disease patterns and initial systemic therapy used. Presented By Seema Khan at TBD

Results: primary tumor characteristics. Presented By Seema Khan at TBD

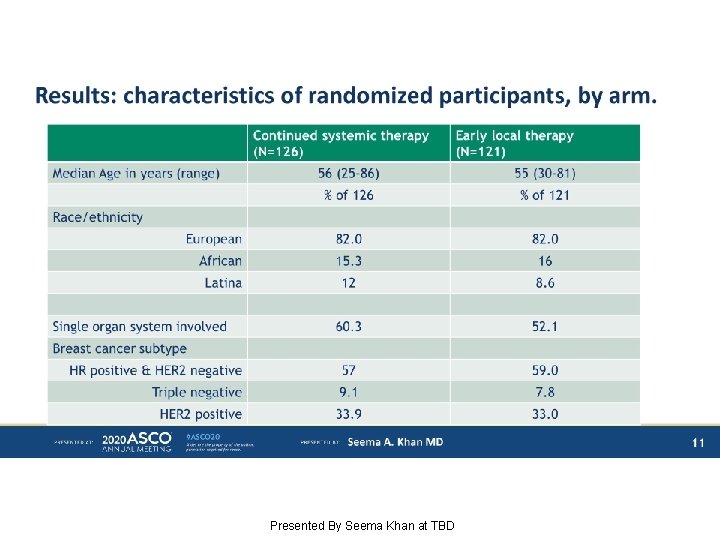
Results: characteristics of randomized participants, by arm. Presented By Seema Khan at TBD

Delivery of locoregional therapy in early local therapy arm Presented By Seema Khan at TBD

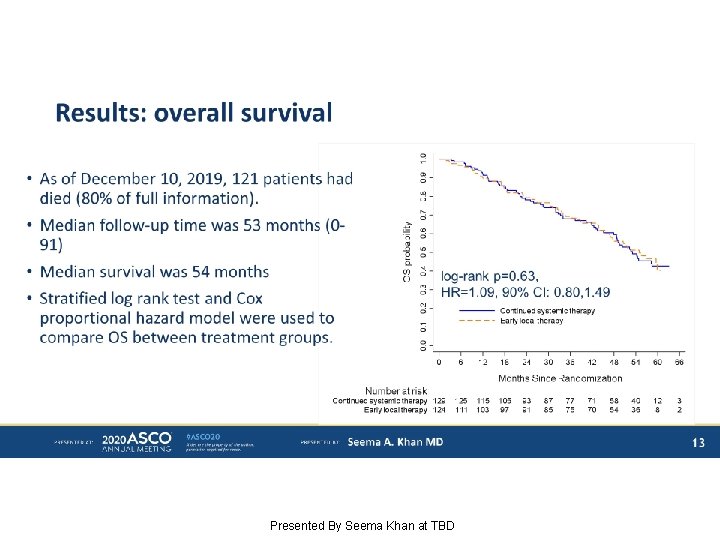
Results: overall survival Presented By Seema Khan at TBD

Results: progression-free survival Presented By Seema Khan at TBD

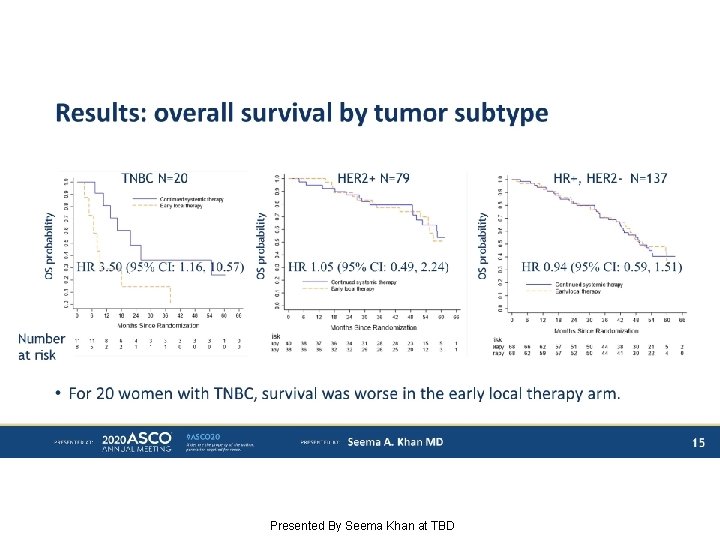
Results: overall survival by tumor subtype Presented By Seema Khan at TBD

Locoregional progression. Presented By Seema Khan at TBD

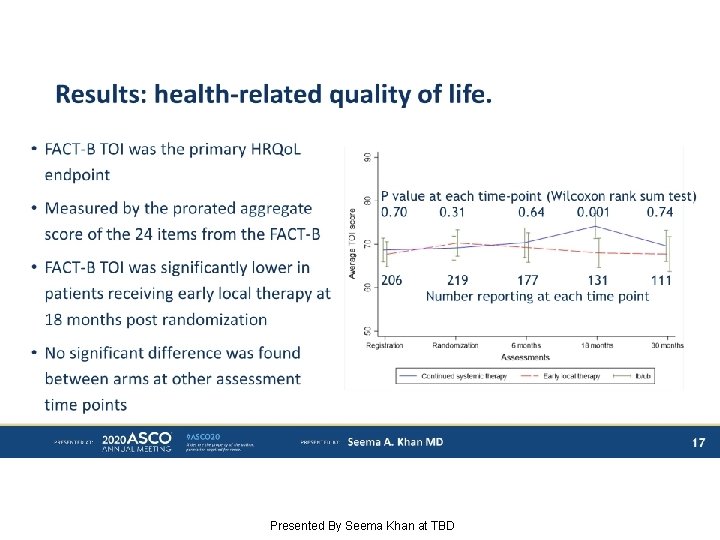
Results: health-related quality of life. Presented By Seema Khan at TBD

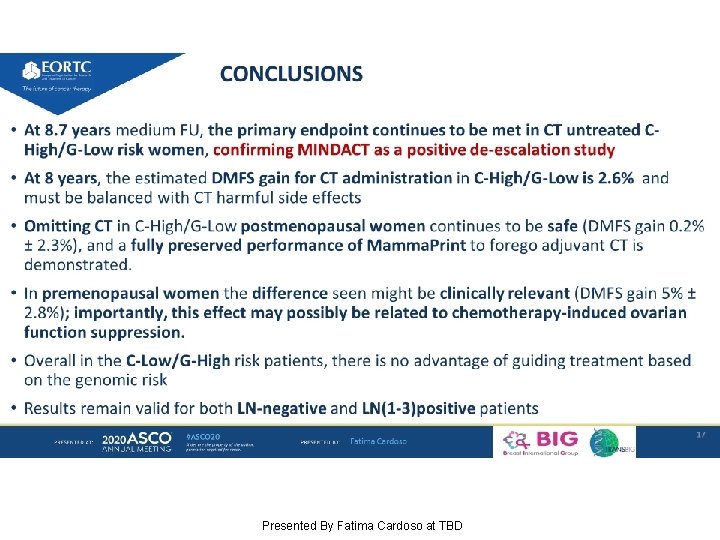
Conclusions Presented By Seema Khan at TBD

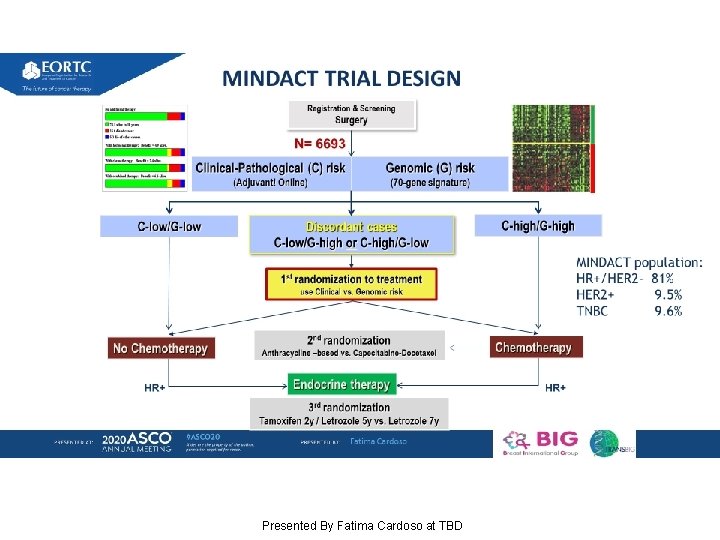
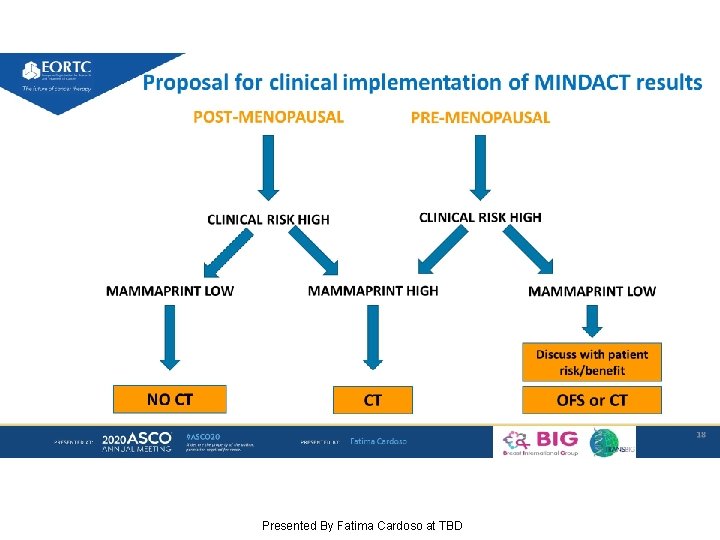
MINDACT: Long-term results of the large prospective trial testing the 70 -gene signature Mamma. Print as guidance for adjuvant chemotherapy in breast cancer patients Presented By Fatima Cardoso at TBD

Slide 2 Presented By Fatima Cardoso at TBD

Slide 3 Presented By Fatima Cardoso at TBD

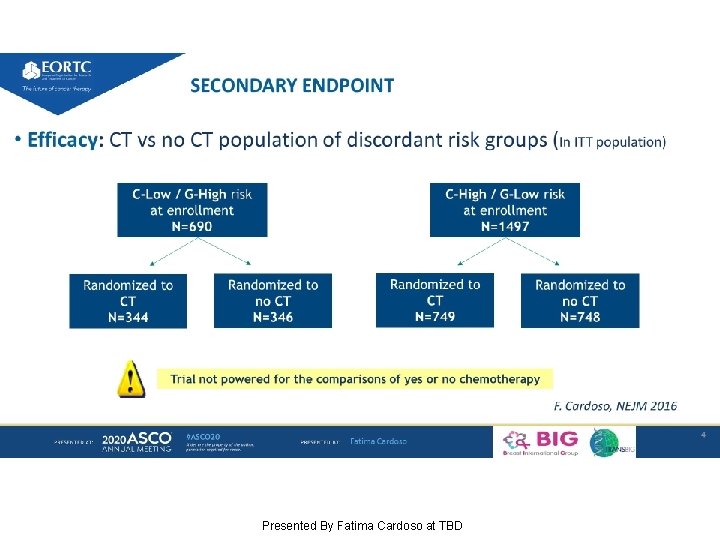
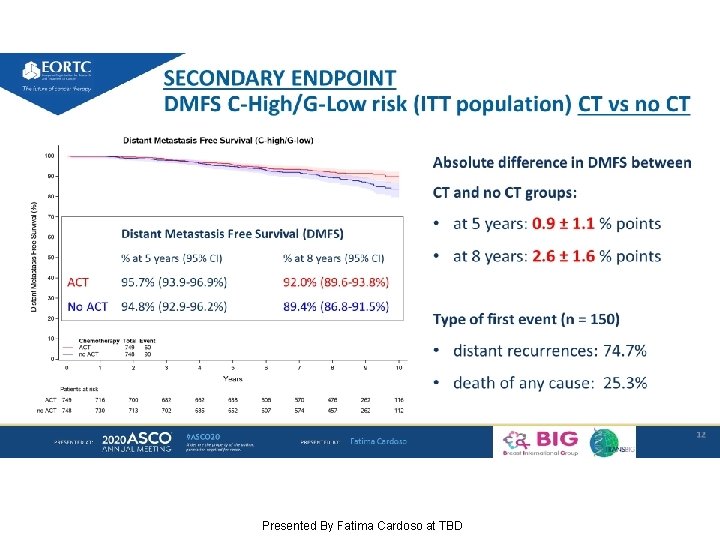
SECONDARY ENDPOINT Presented By Fatima Cardoso at TBD

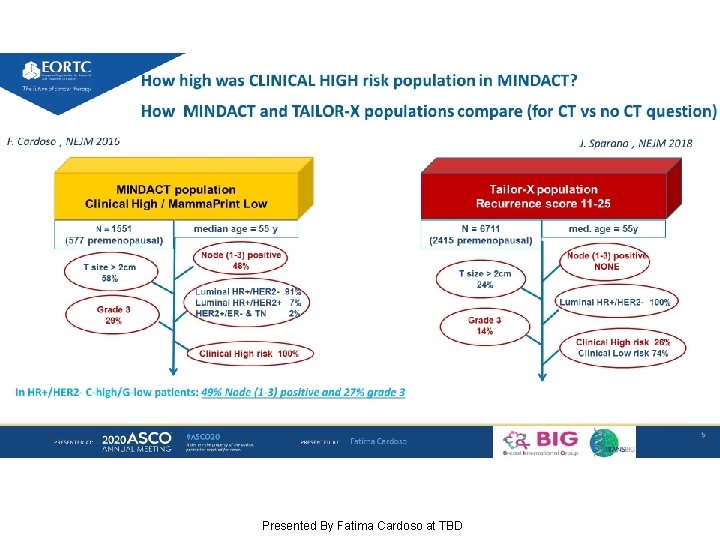
Slide 5 Presented By Fatima Cardoso at TBD

MINDACT successfully met its primary endpoint SUMMARY OF CONCLUSIONS OF PRIMARY ANALYSIS (5 y median FU) Presented By Fatima Cardoso at TBD

UPDATED ANALYSIS AT 8. 7 YEARS MEDIAN FOLLOW-UP RESULTS Presented By Fatima Cardoso at TBD

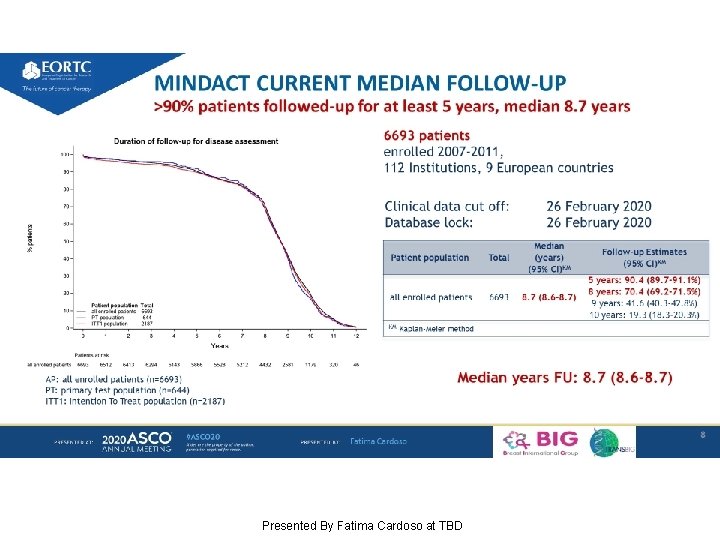
MINDACT CURRENT MEDIAN FOLLOW-UP >90% patients followed-up for at least 5 years, median 8. 7 years Presented By Fatima Cardoso at TBD

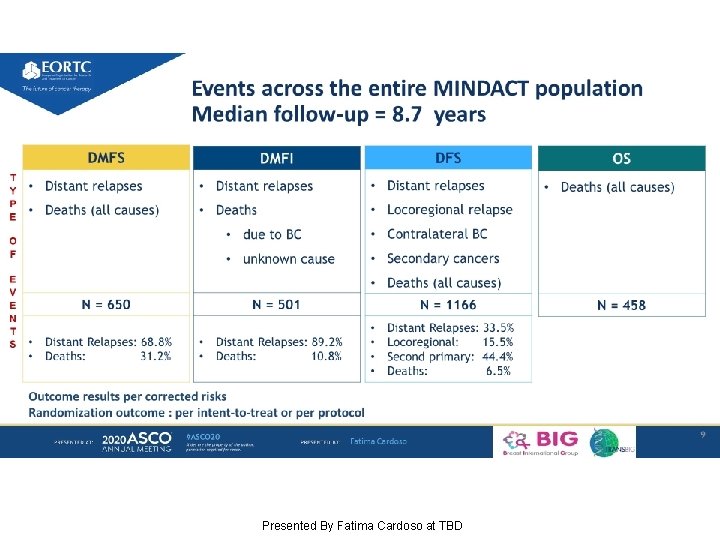
Events across the entire MINDACT population Median follow-up = 8. 7 years Presented By Fatima Cardoso at TBD

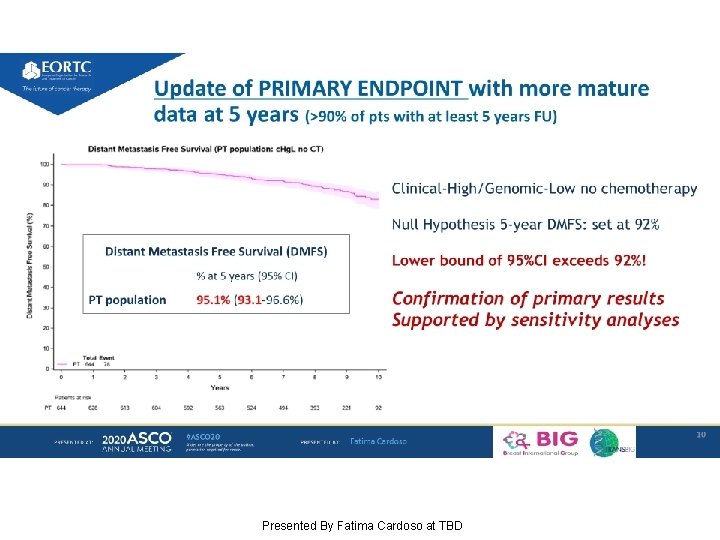
Update of PRIMARY ENDPOINT with more mature data at 5 years (>90% of pts with at least 5 years FU) Presented By Fatima Cardoso at TBD

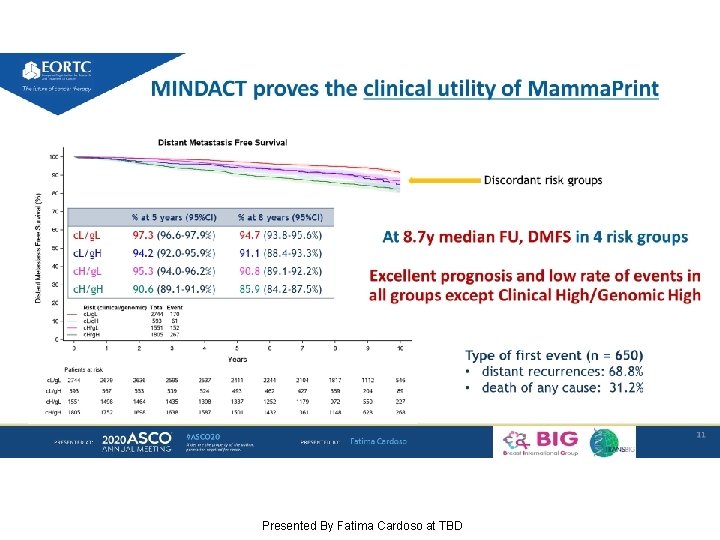
At 8. 7 y median FU, DMFS in 4 risk groups Excellent prognosis and low rate of events in all groups except Clinical High/Genomic High Presented By Fatima Cardoso at TBD

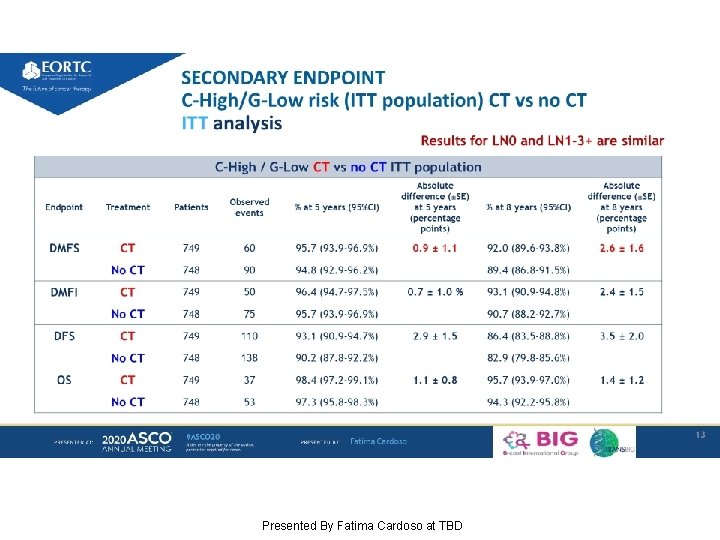
SECONDARY ENDPOINT DMFS C-High/G-Low risk (ITT population) CT vs no CT Presented By Fatima Cardoso at TBD

SECONDARY ENDPOINT C-High/G-Low risk (ITT population) CT vs no CT ITT analysis Presented By Fatima Cardoso at TBD

Effect of chemotherapy by age in HR+/HER 2 - subgroup C-High/G-Low group Presented By Fatima Cardoso at TBD

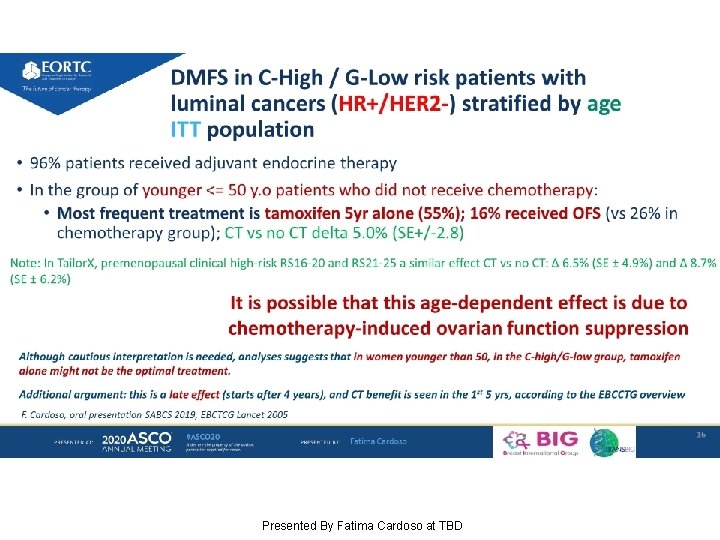
DMFS in C-High / G-Low risk patients with luminal cancers (HR+/HER 2 -) stratified by age ITT population Presented By Fatima Cardoso at TBD

DMFS in C-High / G-Low risk patients with luminal cancers (HR+/HER 2 -) stratified by age ITT population Presented By Fatima Cardoso at TBD

Slide 17 Presented By Fatima Cardoso at TBD

Slide 18 Presented By Fatima Cardoso at TBD

KEYNOTE-355: Randomized, Double-Blind, Phase 3 Study of Pembrolizumab + Chemotherapy versus Placebo + Chemotherapy for Previously Untreated Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer Presented By Javier Cortes at TBD

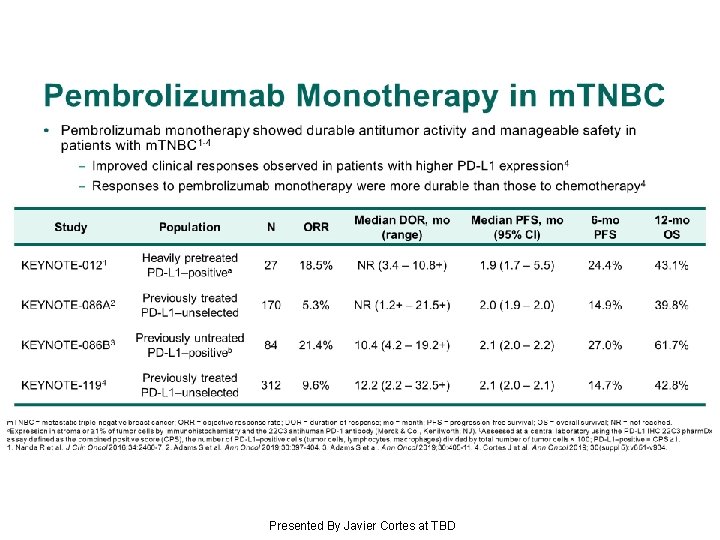
Pembrolizumab Monotherapy in m. TNBC Presented By Javier Cortes at TBD

Pembrolizumab Plus Chemotherapy Presented By Javier Cortes at TBD

KEYNOTE-355 Study Design (NCT 02819518) Presented By Javier Cortes at TBD

Study Endpoints Presented By Javier Cortes at TBD

Statistical Considerations Presented By Javier Cortes at TBD

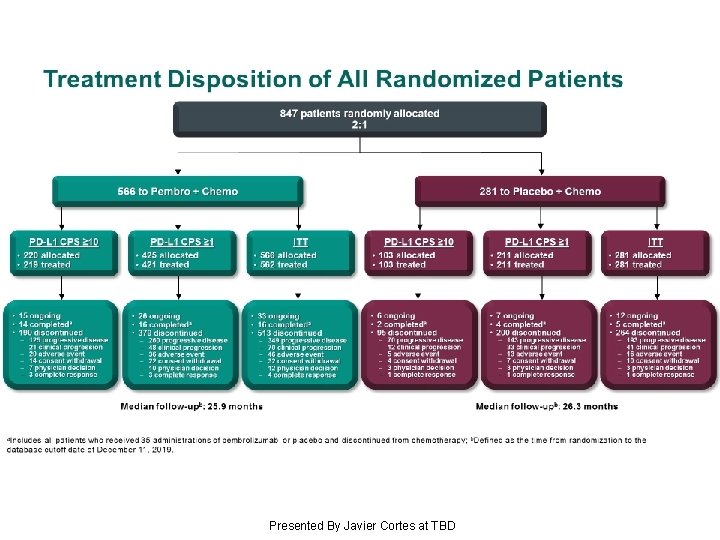
Treatment Disposition of All Randomized Patients Presented By Javier Cortes at TBD

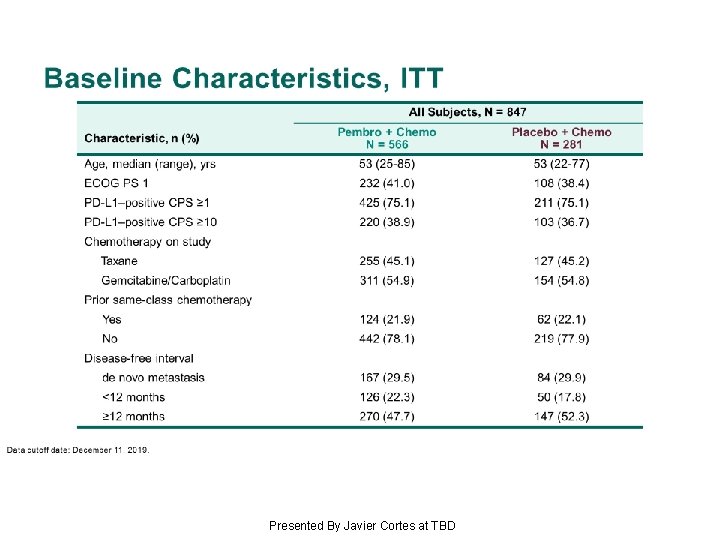
Baseline Characteristics, ITT Presented By Javier Cortes at TBD

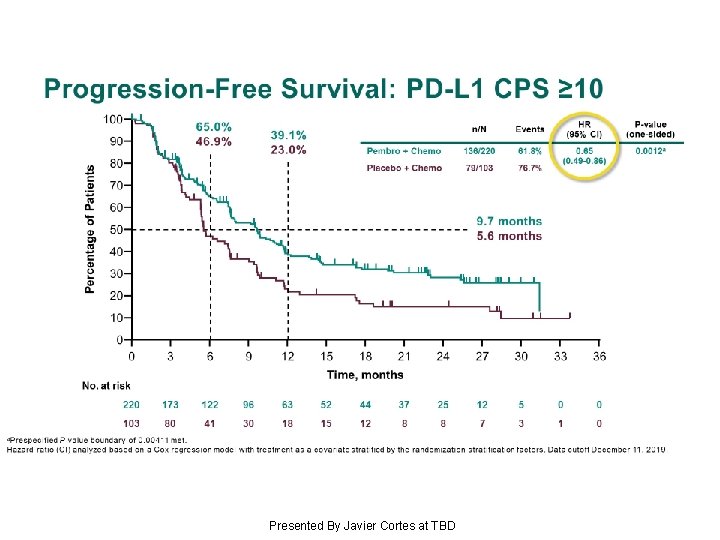
Progression-Free Survival: PD-L 1 CPS ≥ 10 Presented By Javier Cortes at TBD

Progression-Free Survival: PD-L 1 CPS ≥ 10 Presented By Javier Cortes at TBD

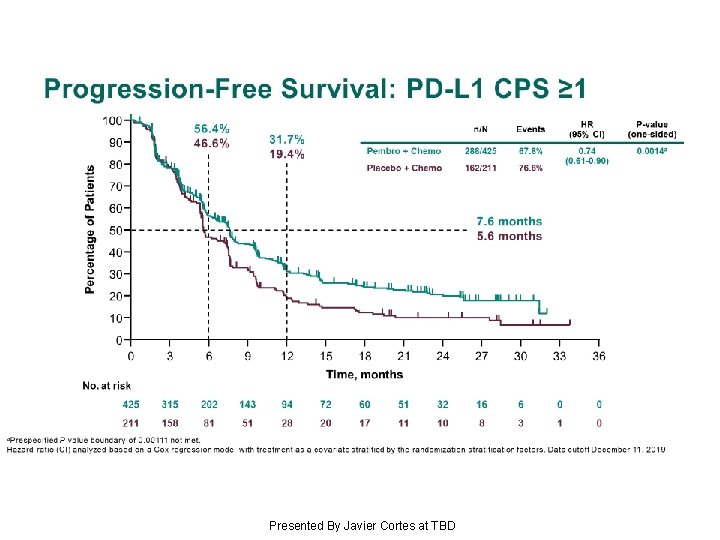
Progression-Free Survival: PD-L 1 CPS ≥ 1 Presented By Javier Cortes at TBD

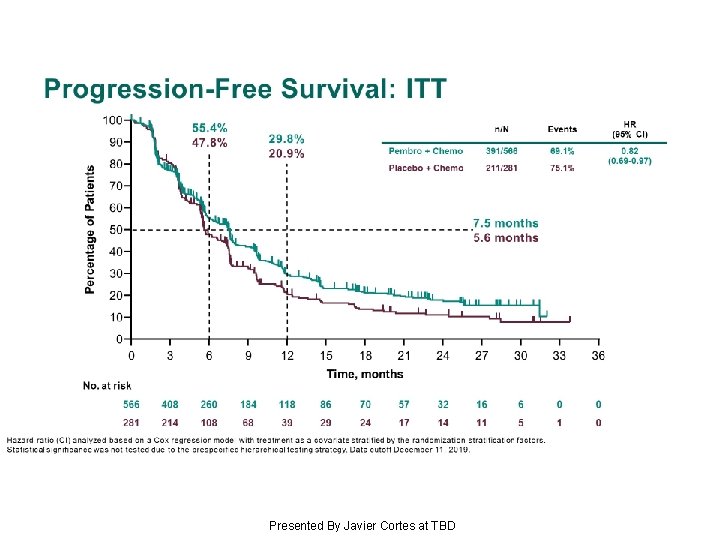
Progression-Free Survival: ITT Presented By Javier Cortes at TBD

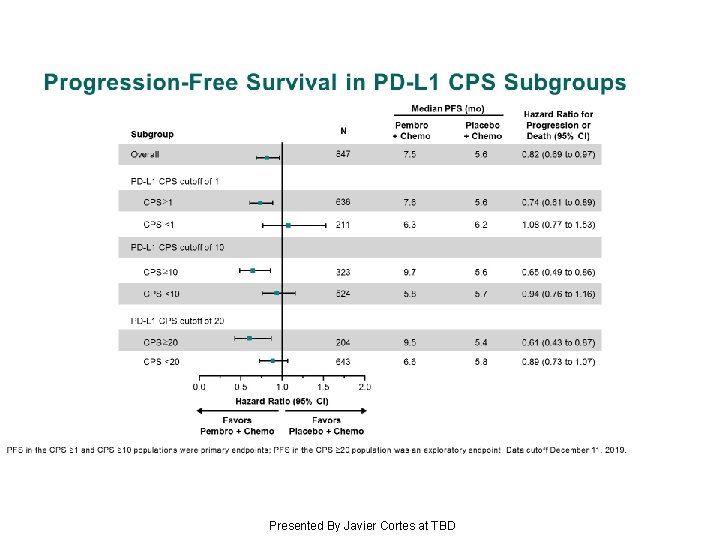
Progression-Free Survival in PD-L 1 CPS Subgroups Presented By Javier Cortes at TBD

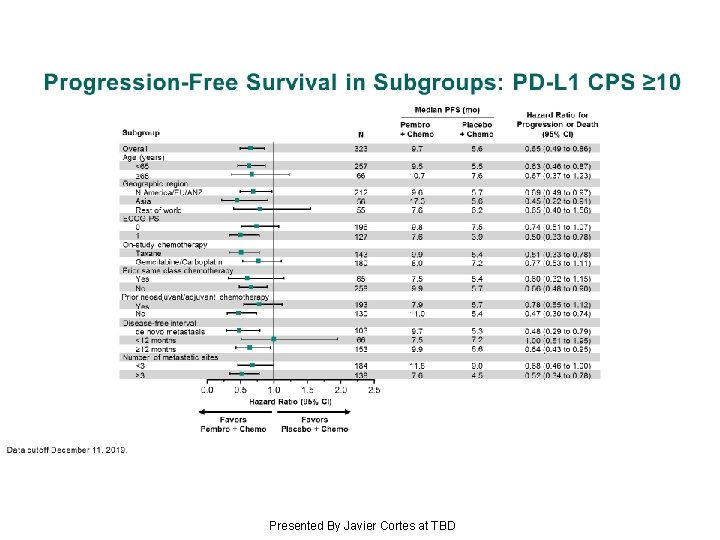
Progression-Free Survival in Subgroups: PD-L 1 CPS ≥ 10 Presented By Javier Cortes at TBD

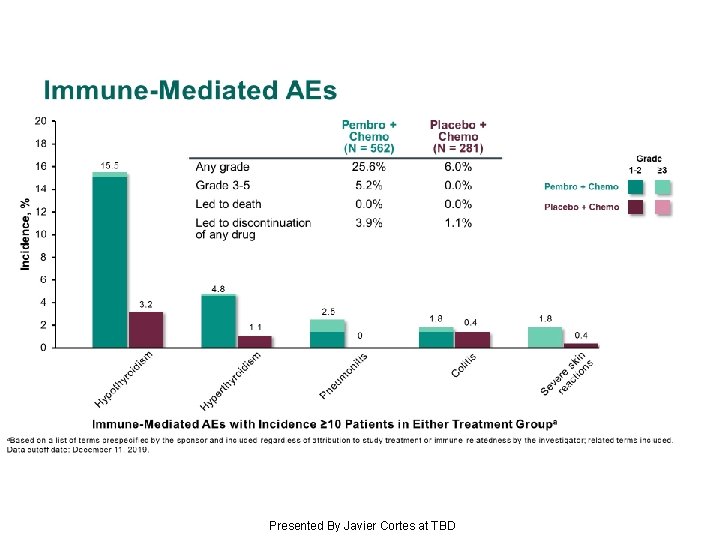
Immune-Mediated AEs Presented By Javier Cortes at TBD

Summary Presented By Javier Cortes at TBD