A Quantum Journey The 20 th Century View


- Slides: 14

A Quantum Journey The 20 th Century View Of The Atom

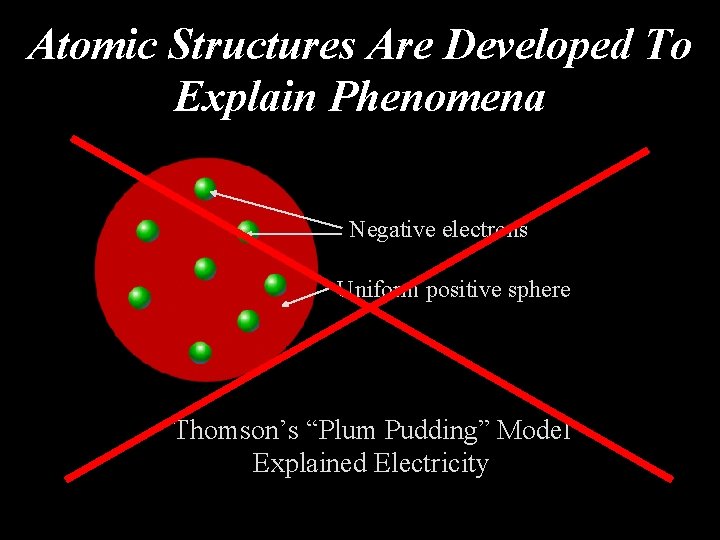
Atomic Structures Are Developed To Explain Phenomena Negative electrons Uniform positive sphere Thomson’s “Plum Pudding” Model Explained Electricity

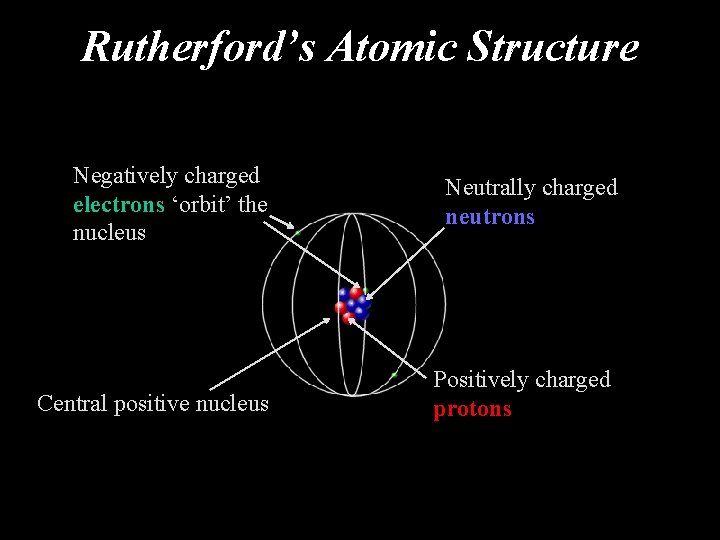
Rutherford’s Atomic Structure Negatively charged electrons ‘orbit’ the nucleus Neutrally charged neutrons Central positive nucleus Positively charged protons

Problem With Rutherford’s Model Accelerated charged particles will radiate EM waves The electron is in orbit; therefore accelerated It should lose energy and spiral inwards to nucleus EM waves But this doesn’t happen!

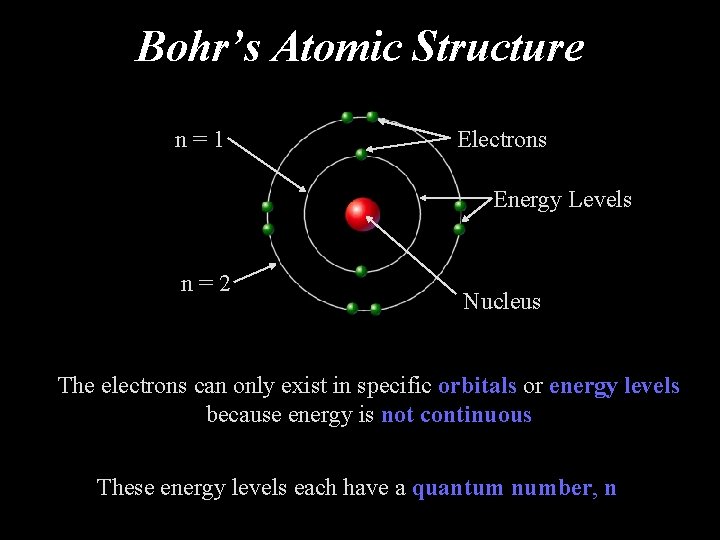
Bohr’s Atomic Structure n=1 Electrons Energy Levels n=2 Nucleus The electrons can only exist in specific orbitals or energy levels because energy is not continuous These energy levels each have a quantum number, n

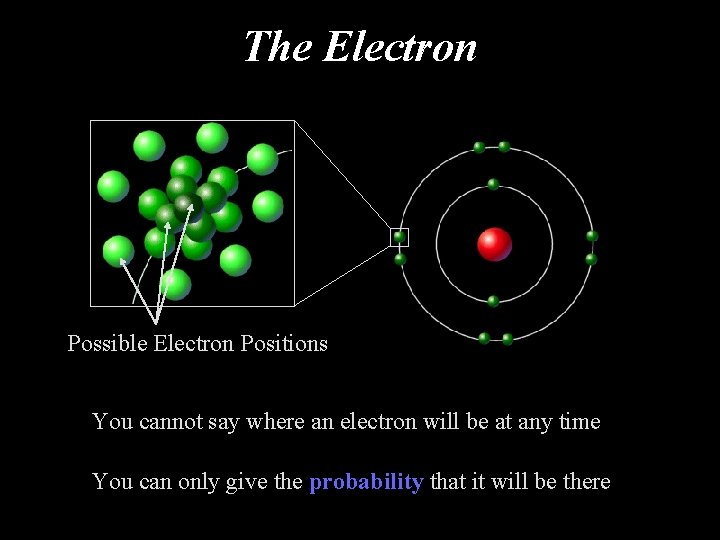
The Electron Possible Electron Positions You cannot say where an electron will be at any time You can only give the probability that it will be there

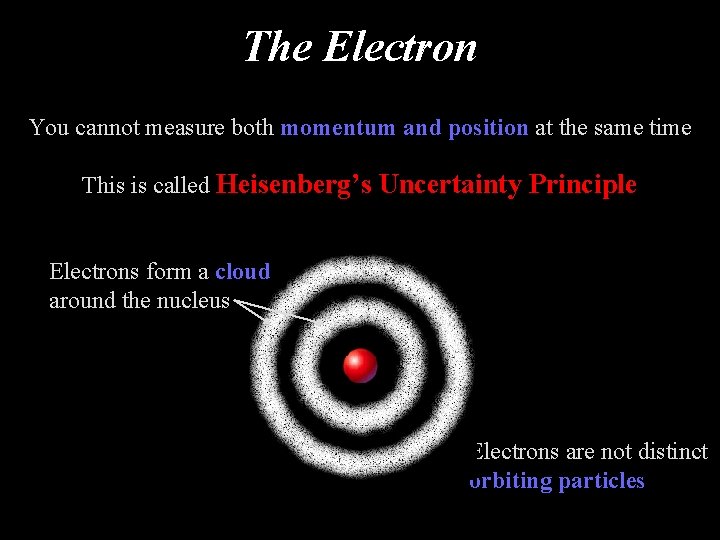
The Electron You cannot measure both momentum and position at the same time This is called Heisenberg’s Uncertainty Principle Electrons form a cloud around the nucleus Electrons are not distinct orbiting particles

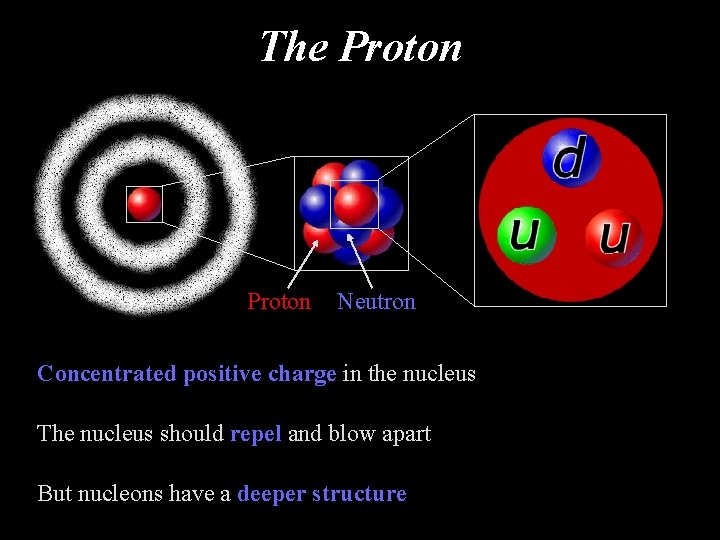
The Proton Neutron Concentrated positive charge in the nucleus The nucleus should repel and blow apart But nucleons have a deeper structure

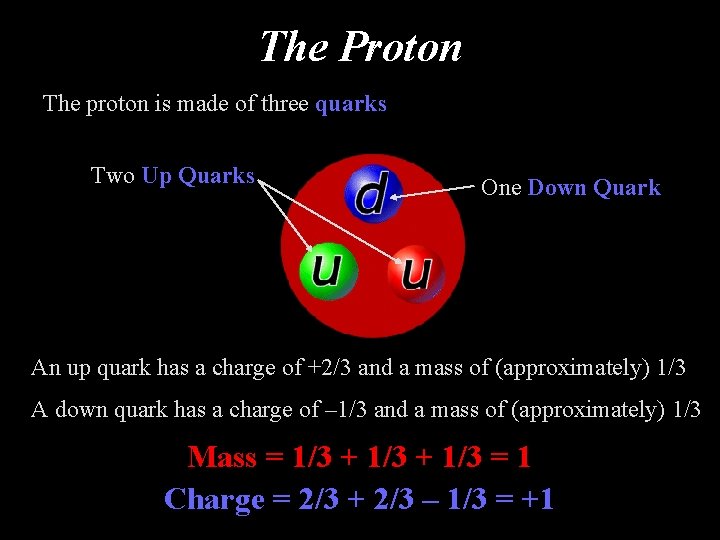
The Proton The proton is made of three quarks Two Up Quarks One Down Quark An up quark has a charge of +2/3 and a mass of (approximately) 1/3 A down quark has a charge of – 1/3 and a mass of (approximately) 1/3 Mass = 1/3 + 1/3 = 1 Charge = 2/3 + 2/3 – 1/3 = +1

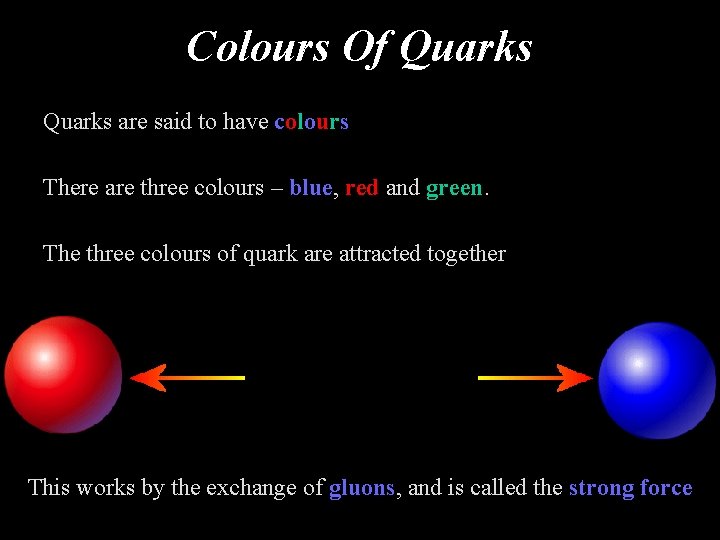
Colours Of Quarks are said to have colours There are three colours – blue, red and green. The three colours of quark are attracted together This works by the exchange of gluons, and is called the strong force

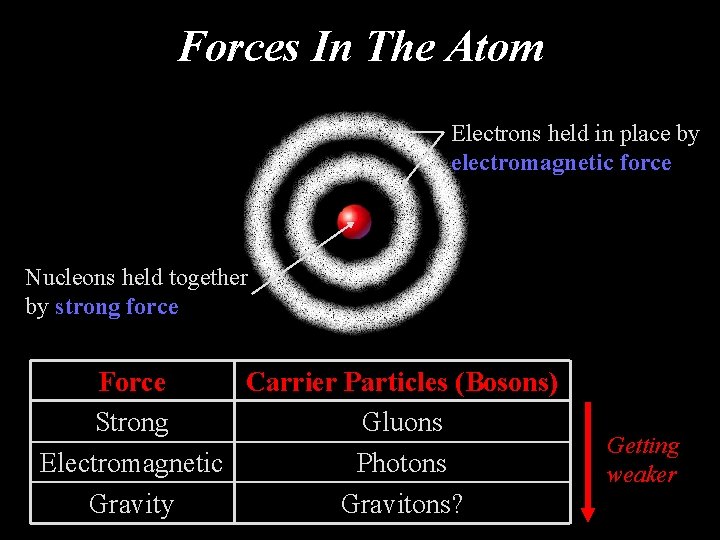
Forces In The Atom Electrons held in place by electromagnetic force Nucleons held together by strong force Force Carrier Particles (Bosons) Strong Gluons Electromagnetic Photons Gravity Gravitons? Getting weaker

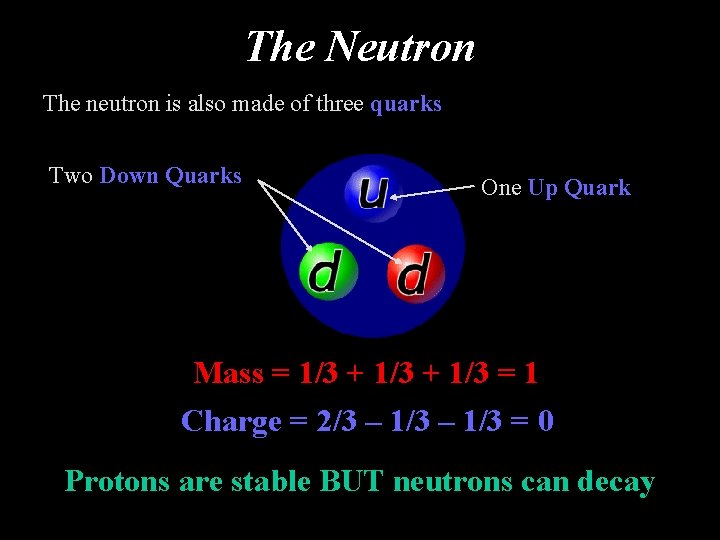
The Neutron The neutron is also made of three quarks Two Down Quarks One Up Quark Mass = 1/3 + 1/3 = 1 Charge = 2/3 – 1/3 = 0 Protons are stable BUT neutrons can decay

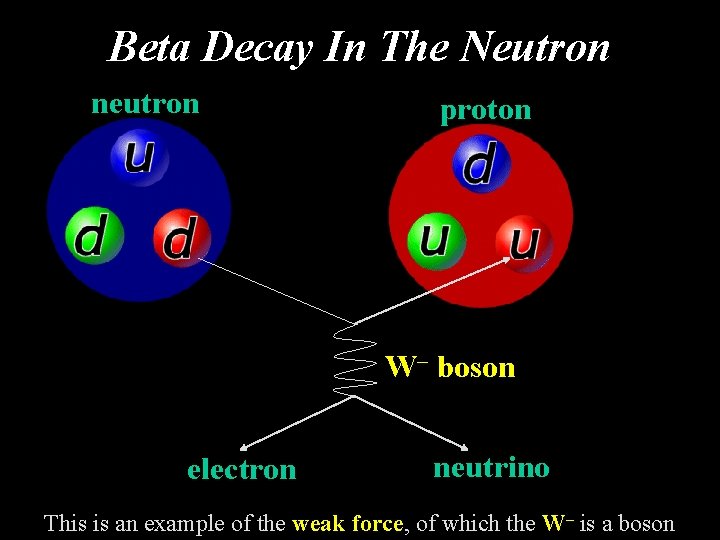
Beta Decay In The Neutron neutron proton W– boson electron neutrino This is an example of the weak force, of which the W– is a boson

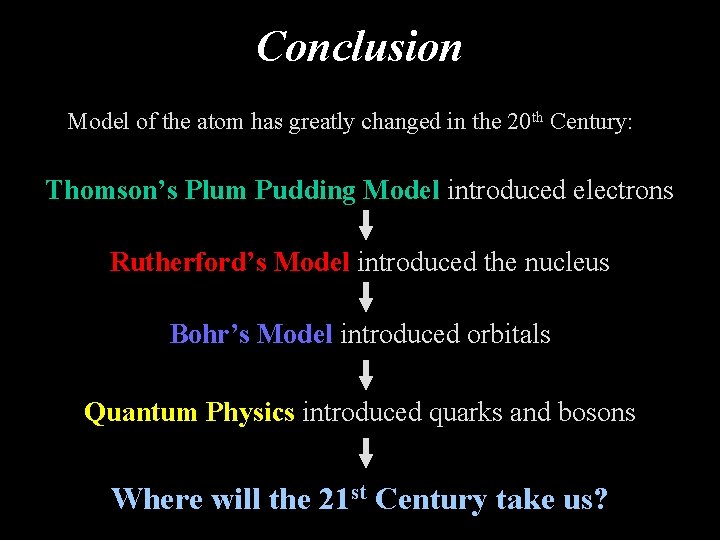
Conclusion Model of the atom has greatly changed in the 20 th Century: Thomson’s Plum Pudding Model introduced electrons Rutherford’s Model introduced the nucleus Bohr’s Model introduced orbitals Quantum Physics introduced quarks and bosons Where will the 21 st Century take us?