A Patient Level Pooled Analysis of Neuro Protection







- Slides: 14

A Patient Level Pooled Analysis of Neuro. Protection with the Tri. Guard Embolic DEFLECTion Device Compared to Unprotected Transcatheter Aortic Valve Replacement Alexandra Lansky, MD Yale University School of Medicine New Haven, CT John Forrest, Adam Brickman, Didier Tchetche, Pieter Stella, Thomas Cuisset, Joachim Schofer, Kevin Abrams, Michael Haude, and Andreas Baumbach

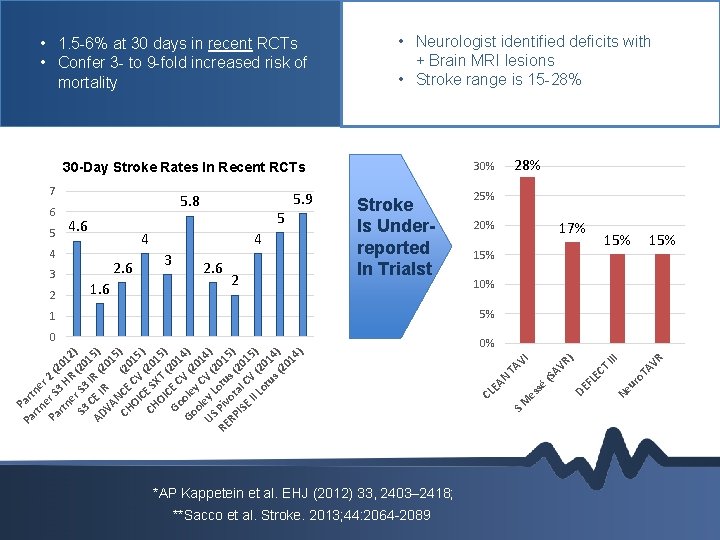
• 1. 5 -6% at 30 days in recent RCTs • Confer 3 - to 9 -fold increased risk of mortality • Neurologist identified deficits with + Brain MRI lesions • Stroke range is 15 -28% 7 4 2. 6 2 17% 15% 15% 10% 5% 1 0 *AP Kappetein et al. EHJ (2012) 33, 2403– 2418; **Sacco et al. Stroke. 2013; 44: 2064 -2089 R o. T AV ur Ne T EC DE FL AV R (S sé III ) I P Pa art rtn ne e r Pa r S 2 (2 rtn 3 H 01 e R 2) S 3 r S 3 (20 1 AD CE I IR (2 5) 01 VA R NC ( 5) CH E 20 OI CV 15 ) C CH E S (201 OI XT 5) C ( Go E C 201 Go ole V (2 4) ol y C 01 US ey L V ( 4) 2 RE Piv otu 015 s o RP t ) IS al C (20 1 E II V ( 5) Lo 20 tu 14 s( ) 20 14 ) 0% es 1. 6 3 20% TA V 2 2. 6 4 25% SM 4 3 5 Stroke Is Underreported In Trialst AN 5 4. 6 5. 9 CL E 6 5. 8 28% 30 -Day Stroke Rates In Recent RCTs

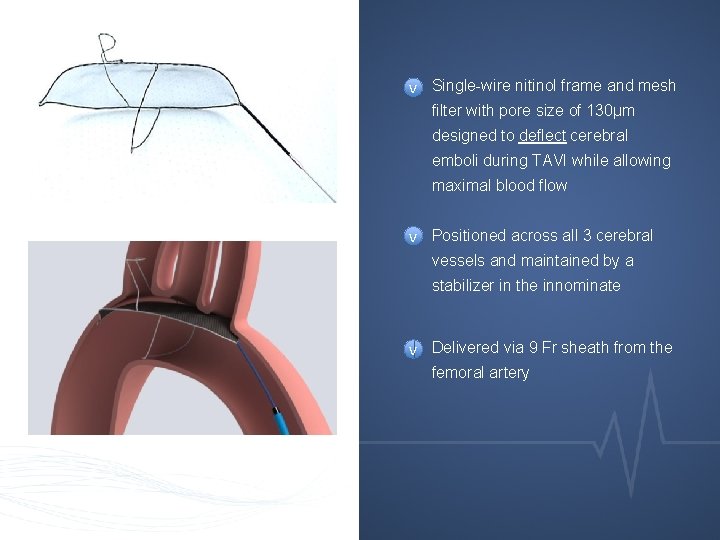
v Single-wire nitinol frame and mesh filter with pore size of 130μm designed to deflect cerebral emboli during TAVI while allowing maximal blood flow v Positioned across all 3 cerebral vessels and maintained by a stabilizer in the innominate v Delivered via 9 Fr sheath from the femoral artery

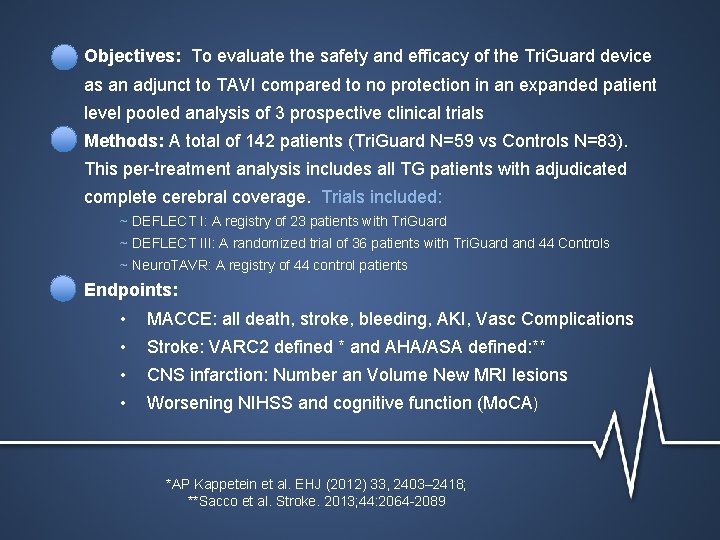
Objectives: To evaluate the safety and efficacy of the Tri. Guard device as an adjunct to TAVI compared to no protection in an expanded patient level pooled analysis of 3 prospective clinical trials Methods: A total of 142 patients (Tri. Guard N=59 vs Controls N=83). This per-treatment analysis includes all TG patients with adjudicated complete cerebral coverage. Trials included: ~ DEFLECT I: A registry of 23 patients with Tri. Guard ~ DEFLECT III: A randomized trial of 36 patients with Tri. Guard and 44 Controls ~ Neuro. TAVR: A registry of 44 control patients Endpoints: • MACCE: all death, stroke, bleeding, AKI, Vasc Complications • Stroke: VARC 2 defined * and AHA/ASA defined: ** • CNS infarction: Number an Volume New MRI lesions • Worsening NIHSS and cognitive function (Mo. CA) *AP Kappetein et al. EHJ (2012) 33, 2403– 2418; **Sacco et al. Stroke. 2013; 44: 2064 -2089

All 3 Trials Used Same Methodology, Definitions, MRI Core Lab & CEC Screening Procedure Post-Procedure 4± 2 days NIHSS m. RS Neurocog* TAVR ± Tri. Guard 30 Days +30 days DW-MRI NIHSS m. RS Neurocog* *Neurocognitive test battery includes the Montreal Cognitive Assessment (Mo. CA) and computerized Cog. State Research Test. Baseline and 30 -day evaluations include supplemental Digit Symbol Substitution, Trailmaking, and Word Fluency Tests.

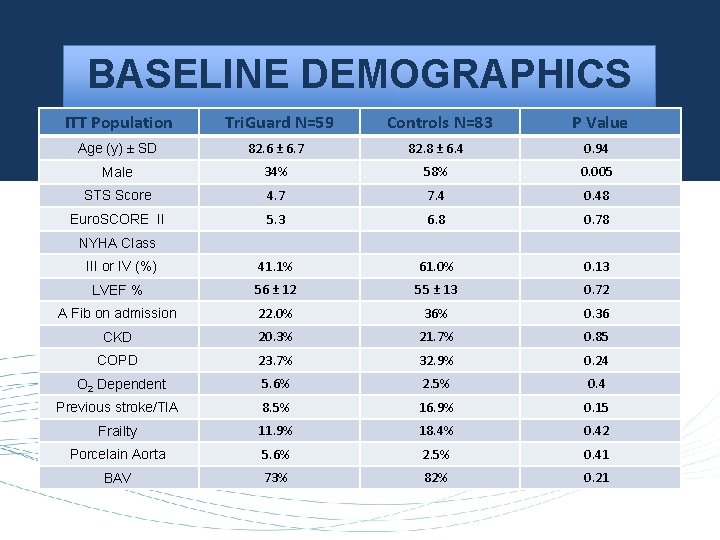
BASELINE DEMOGRAPHICS ITT Population Tri. Guard N=59 Controls N=83 P Value Age (y) ± SD 82. 6 ± 6. 7 82. 8 ± 6. 4 0. 94 Male 34% 58% 0. 005 STS Score 4. 7 7. 4 0. 48 Euro. SCORE II 5. 3 6. 8 0. 78 III or IV (%) 41. 1% 61. 0% 0. 13 LVEF % 56 ± 12 55 ± 13 0. 72 A Fib on admission 22. 0% 36% 0. 36 CKD 20. 3% 21. 7% 0. 85 COPD 23. 7% 32. 9% 0. 24 O 2 Dependent 5. 6% 2. 5% 0. 4 Previous stroke/TIA 8. 5% 16. 9% 0. 15 Frailty 11. 9% 18. 4% 0. 42 Porcelain Aorta 5. 6% 2. 5% 0. 41 BAV 73% 82% 0. 21 NYHA Class

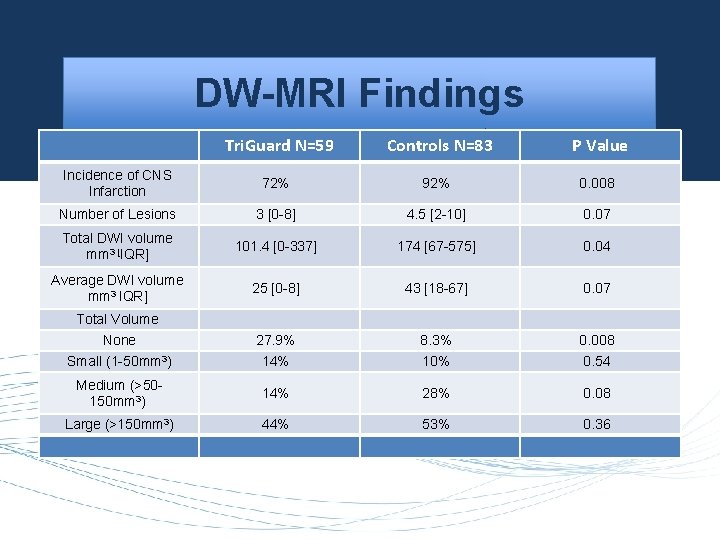
DW-MRI Findings Tri. Guard N=59 Controls N=83 P Value Incidence of CNS Infarction 72% 92% 0. 008 Number of Lesions 3 [0 -8] 4. 5 [2 -10] 0. 07 Total DWI volume mm 3 [IQR] 101. 4 [0 -337] 174 [67 -575] 0. 04 Average DWI volume mm 3 IQR] 25 [0 -8] 43 [18 -67] 0. 07 27. 9% 14% 8. 3% 10% 0. 008 0. 54 Medium (>50150 mm 3) 14% 28% 0. 08 Large (>150 mm 3) 44% 53% 0. 36 Total Volume None Small (1 -50 mm 3)

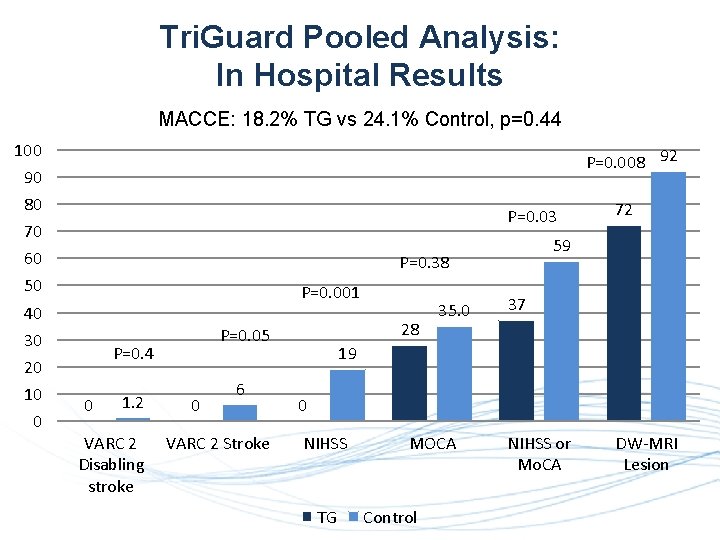
Tri. Guard Pooled Analysis: In Hospital Results MACCE: 18. 2% TG vs 24. 1% Control, p=0. 44 100 P=0. 008 92 90 80 P=0. 03 70 60 P=0. 001 40 30 0 0 1. 2 VARC 2 Disabling stroke 28 P=0. 05 P=0. 4 20 10 59 P=0. 38 50 0 6 VARC 2 Stroke 35. 0 72 37 19 0 NIHSS TG MOCA Control NIHSS or Mo. CA DW-MRI Lesion

C O N C L U S I O N S Neuroprotection with Tri. Guard Ø Is safe Ø Significantly reduced in hospital VARC 2 defined stroke (0% vs. 6. 0%, p=0. 05) Ø Significantly reduced stroke rate defined by worsening NIHSS (National Institutes of Health Stroke Scale) with DW-MRI lesions (0% vs. 19%, p=0. 002) Ø Tri. Guard protected patients showed higher absence of CNS infarction (28% vs. 8%, p=0. 008) Ø Total lesion volume was reduced significantly with Tri. Guard vs. no protection (101. 4 mm 3 vs. 174. 0 mm 3, p=0. 04) Ø None of the Tri. Guard protected patients had worsened NIHS score, while 17. 1% of the patients without protection had worsened NIHS score (p=0. 001) These clinically meaningful outcomes, clearly demonstrate the importance of using Tri. Guard, and the potential consequences of unprotected procedures. The pivotal REFLECT RCT is designed to confirm our results.

Clinical Outcomes of the Keystone Heart rd 3 Generation deflection Device Prof J Schofer, MD, Ph. D PCR 2016; Euro 16 A-OP 0098 - Stella P. , Abawi M. , Voskuil M. , Bijuklic K. , Riess F. , Hansen L. , Schofer J.

Aims The Tri. Guard cerebral embolic protection System is a nitinol frame and mesh that is positioned in the aortic arch to prevent cerebral embolization. We evaluated the Clinical performance of the third Generation Tri. Guard device in 2 European centers.

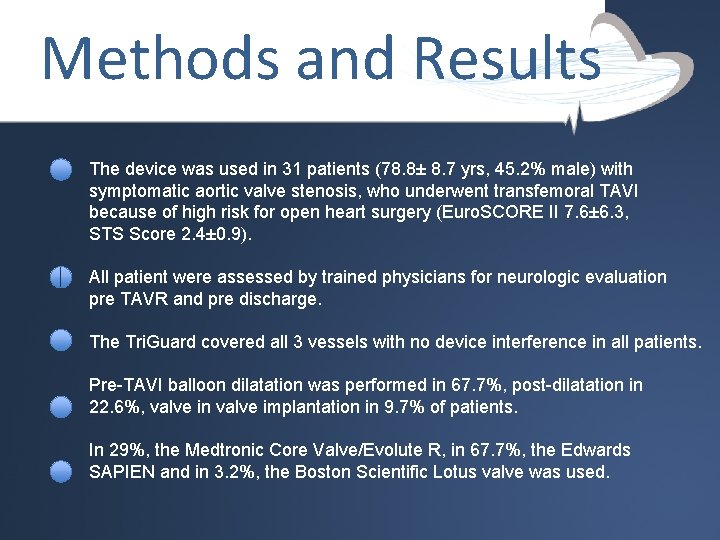
Methods and Results The device was used in 31 patients (78. 8± 8. 7 yrs, 45. 2% male) with symptomatic aortic valve stenosis, who underwent transfemoral TAVI because of high risk for open heart surgery (Euro. SCORE II 7. 6± 6. 3, STS Score 2. 4± 0. 9). All patient were assessed by trained physicians for neurologic evaluation pre TAVR and pre discharge. The Tri. Guard covered all 3 vessels with no device interference in all patients. Pre-TAVI balloon dilatation was performed in 67. 7%, post-dilatation in 22. 6%, valve in valve implantation in 9. 7% of patients. In 29%, the Medtronic Core Valve/Evolute R, in 67. 7%, the Edwards SAPIEN and in 3. 2%, the Boston Scientific Lotus valve was used.

Safety (VARC 2) Results show ~ MACCE 9. 7% (3/31). ~ Mortality, 0. 0% (0/31) ~ All stroke, 0. 0% (0/31) disabling stroke, 0. 0% (0/31); non-disabling stroke, 0. 0% (0/31). ~ Life-threatening or disabling bleeding, 9. 7% (3/31) and device related bleeding, 0. 0% (0/31). Tri. Guard related, 0. 0% (0/31). ~ AKI Stage 2/3, 0. 0% (0/31). ~ Major Vascular Complication, 0. 0% (0/31). This study is currently ongoing and we are looking forward to further updates in upcoming meetings.

Conclusions The third generation Tri. Guard device is safe with improved performance and ease of use, 100% of the cases had 3 vessel coverage and no strokes.
 Pooled time series
Pooled time series Standard error of difference
Standard error of difference Kruskal wallis test formula
Kruskal wallis test formula Pooled variance
Pooled variance Pooled variance estimate formula
Pooled variance estimate formula Pooled interdependence
Pooled interdependence Pooled standard deviation
Pooled standard deviation Contoh data pooling
Contoh data pooling Rdp sdp difference
Rdp sdp difference Division of florida condominiums
Division of florida condominiums Contoh mediating technology
Contoh mediating technology Subjects predicates
Subjects predicates Pooled negotiating power
Pooled negotiating power Ability 360 locations
Ability 360 locations Pooled negotiating power
Pooled negotiating power