A Number vs Quantity Quantity number unit UNITS

A. Number vs. Quantity • Quantity - number + unit UNITS MATTER!!

Metrics – Base Units Measurement Base Unit Abbreviation Length meter m Mass gram* g Volume liter L Temperature Celcius* (centigrade*) or Kelvin o. C or K *These are not SI units, but are base units for these measurements.
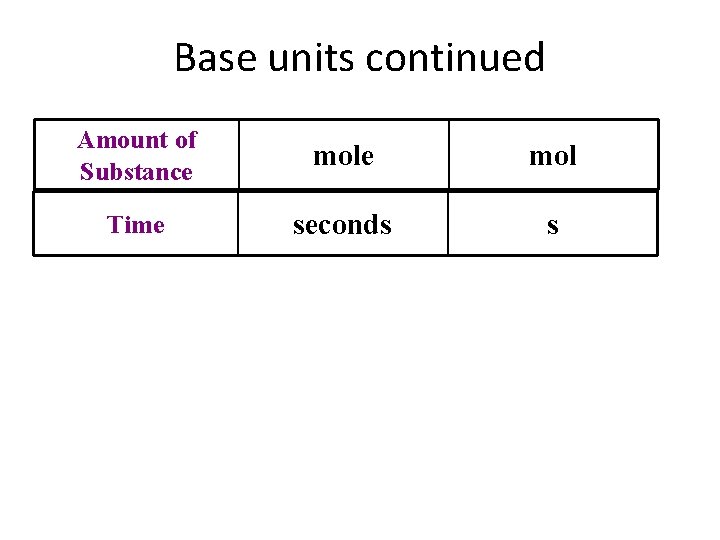
Base units continued Amount of Substance mol Time seconds s
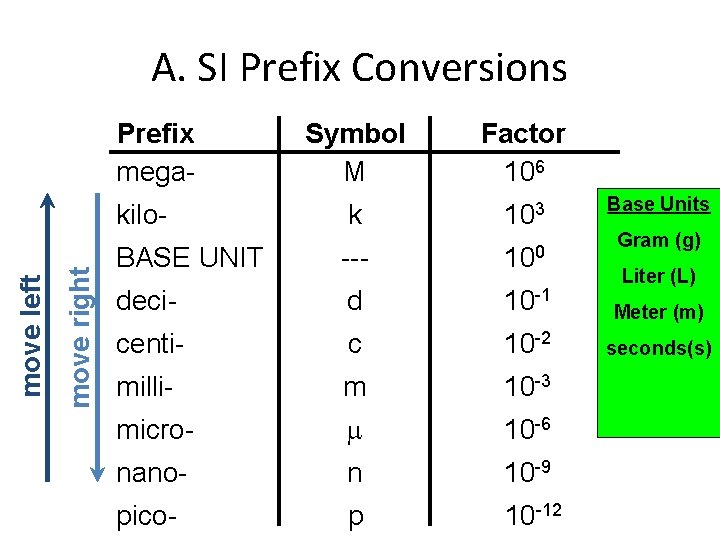
A. SI Prefix Conversions Prefix mega- Symbol M Factor 106 k 103 BASE UNIT --- 100 deci- d 10 -1 Meter (m) centi- c 10 -2 seconds(s) milli- m 10 -3 micro- 10 -6 nano- n 10 -9 pico- p 10 -12 move right move left kilo- Base Units Gram (g) Liter (L)

Metrics Why use the metric system?

Metrics • Because of the worldwide use of science, an international system of measurements was developed in 1960. – This system is referred to as the SI (le Systeme International). • This system utilizes specific SI base units. • The advantage of the system is that it is a decimal system based on multiples of 10. • The system uses prefixes to indicate the multiples of 10. (How many times you move the decimal. )
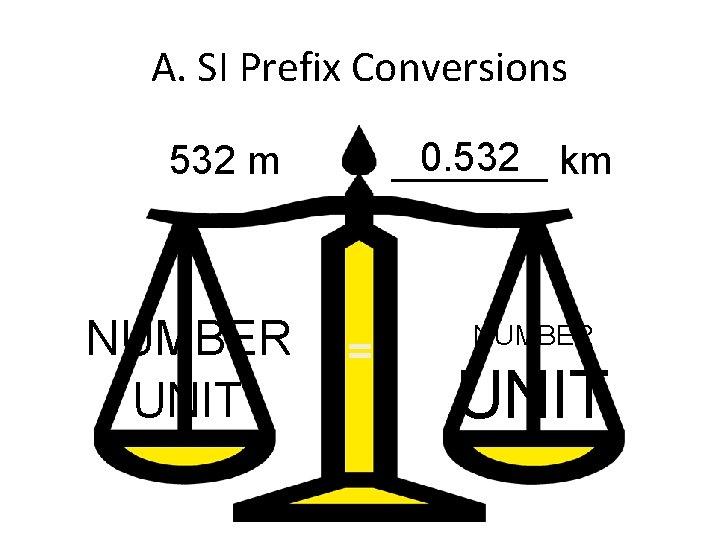
A. SI Prefix Conversions 532 m NUMBER UNIT 0. 532 km = _______ = NUMBER UNIT
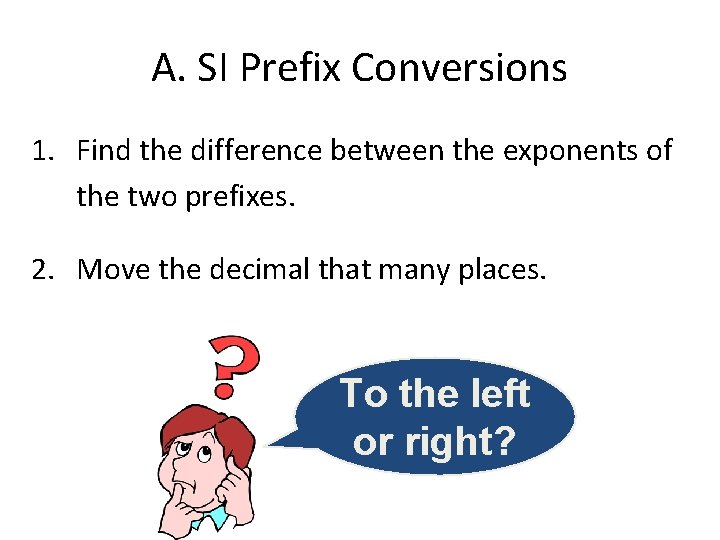
A. SI Prefix Conversions 1. Find the difference between the exponents of the two prefixes. 2. Move the decimal that many places. To the left or right?

(exponent of start)-(exponent of end) • 33 kg to mg – Kilo has an exponent of 3 and milli has an exponent of -3 (3) – (-3) = 6 since it is positive, you move it places to the right • 24 mm to m – Milli has an exponent of -3 and meter is a base unit so it has an exponent of 0 (-3) – (0) = -3 since it is negative you move 3 places to the left
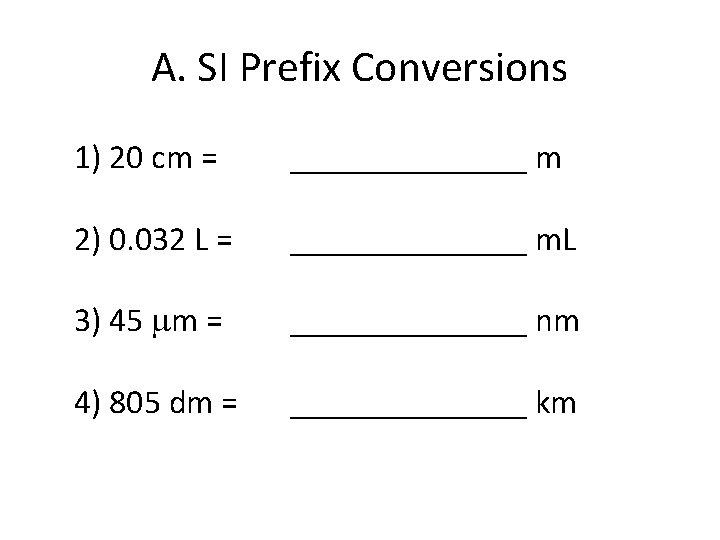
A. SI Prefix Conversions 1) 20 cm = _______ m 2) 0. 032 L = _______ m. L 3) 45 m = _______ nm 4) 805 dm = _______ km
Temperature Scales • Fahrenheit: Based upon the boiling and melting points of alcohol. • Celsius: Based upon the boiling and melting points of water. – 0 o C = Melting Point – 100 o C = Boiling Point 12/22/2021 11
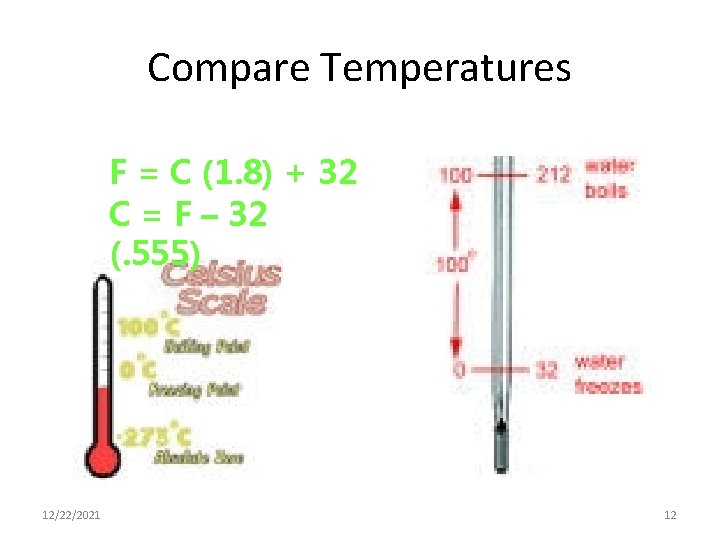
Compare Temperatures F = C (1. 8) + 32 C = F – 32 (. 555) 12/22/2021 12
Absolute Temperature • Kelvin: Based scale upon absolute zero. – Absolute Zero: The coldest temperature possible. (Theoretical Point) • Remember that temperature is based on average KE. Therefore, at 0 Kelvin there will be absolutely no movement of the atoms/molecules of the substance. – Absolute Zero = -273. 16 C – Used the same sized graduations as in the Celsius scale. 12/22/2021 13
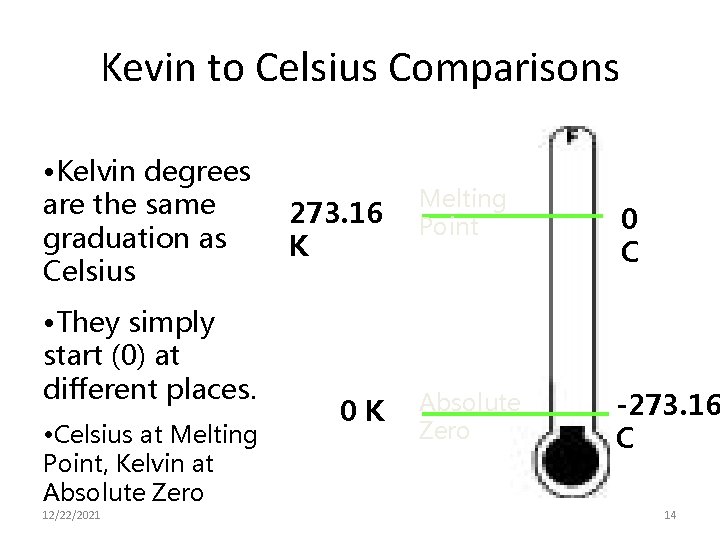
Kevin to Celsius Comparisons • Kelvin degrees are the same graduation as Celsius • They simply start (0) at different places. • Celsius at Melting Point, Kelvin at Absolute Zero 12/22/2021 273. 16 K 0 K Melting Point Absolute Zero 0 C -273. 16 C 14
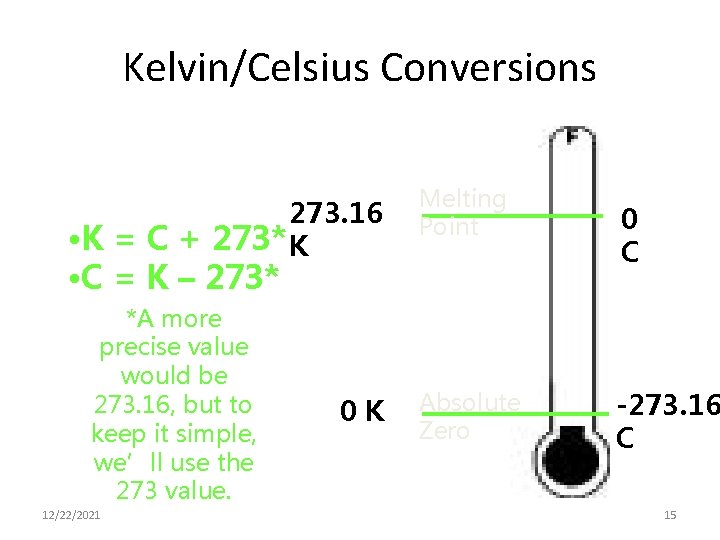
Kelvin/Celsius Conversions 273. 16 • K = C + 273* K Melting Point • C = K – 273* *A more precise value would be 273. 16, but to keep it simple, we’ll use the 273 value. 12/22/2021 0 K Absolute Zero 0 C -273. 16 C 15
- Slides: 15