A Novel Solid Catalyst Process for Biodiesel Production

A Novel Solid Catalyst Process for Biodiesel Production Dheeban Kannan Jack V. Matson June 25, 2008 12 th Annual Green Chemistry and Engineering Conference Washington, DC

Introduction n Biodiesel – fatty acid (m)ethyl esters n Importance – energy environmental economy separation hassles 2 Motivation • Soluble liquid catalysts --> Solid catalysts n
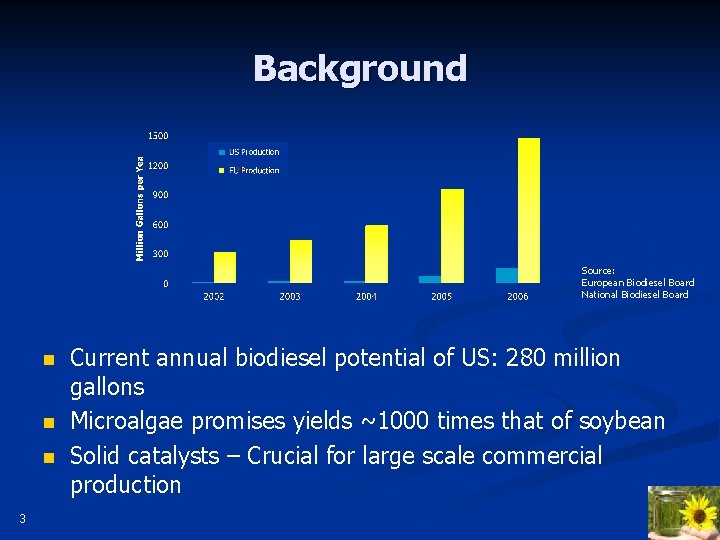
Background Source: European Biodiesel Board National Biodiesel Board n n n 3 Current annual biodiesel potential of US: 280 million gallons Microalgae promises yields ~1000 times that of soybean Solid catalysts – Crucial for large scale commercial production
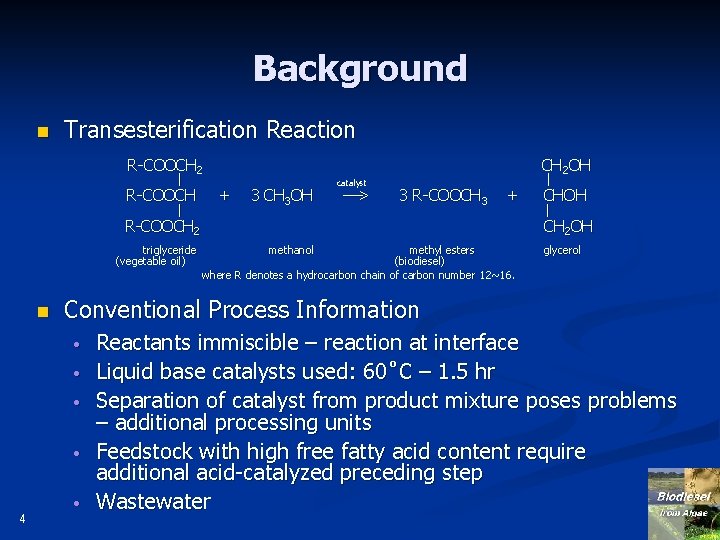
Background n Transesterification Reaction R-COOCH 2 l R-COOCH 2 triglyceride (vegetable oil) n 3 CH 3 OH > 3 R-COOCH 3 methanol + methyl esters (biodiesel) where R denotes a hydrocarbon chain of carbon number 12~16. glycerol Conventional Process Information • • 4 + catalyst CH 2 OH l CH 2 OH • Reactants immiscible – reaction at interface Liquid base catalysts used: 60˚C – 1. 5 hr Separation of catalyst from product mixture poses problems – additional processing units Feedstock with high free fatty acid content require additional acid-catalyzed preceding step Wastewater

Lead-up to Current Research n Solid bases not effective for conventional reaction conditions n Solid acids even behind in performance n Supercritical process 1 • • • 5 No catalyst, 350˚C, 500 atm, 4 min 40: 1 Alcohol: Oil Molar ratio [conventional 6: 1] Pressure too high, Thermal breakdown issues Critical Point: Methanol 240˚C, 80 atm, Ethanol 247˚C, 65 atm With cosolvent 2, 280˚C, 130 atm, 10 min [1]. Saka, S. and Kusdiana, D. , Fuel (2001) 225 [2]. Cao et al. , Fuel 84 (2005) 347

Lead-up to Current Research n Critical regime desired for better miscibility • Alcohol both as a reactant and as a cosolvent n Could mild solid bases be effective near critical regime? n Packed-Bed Reactor - High T okay for continuous process n 6 Suppes et al. 3 tried calcium carbonate • 260˚C, 70 atm, residence time 18 min • 50% biodiesel as cosolvent, otherwise poor yield [3]. Suppes et. al. , J. Am. Oil Chem. Soc. , (2001) 139
Batch Tests 7 n Batch reactors used initially to test and screen catalysts n Various weak solid base salts and mild metal oxide bases were tested n Analytical Procedures: Gas Chromatography (GC) Thin Layer Chromatography (TLC) n Certain metal oxides showed good results (Patent Pending) • ~95% conversion in 18 min n Free fatty acid conversion of 93% observed. Probably due to the amphoteric nature of metal oxides. Eliminates the need of a separate acid-catalyzed preceding step.

Performance of Catalysts – Batch Tests 40: 1 Alcohol: Oil Molar ratio 8 * Catalysts tested at 220, 200, 175, 150 ˚C – similar trend
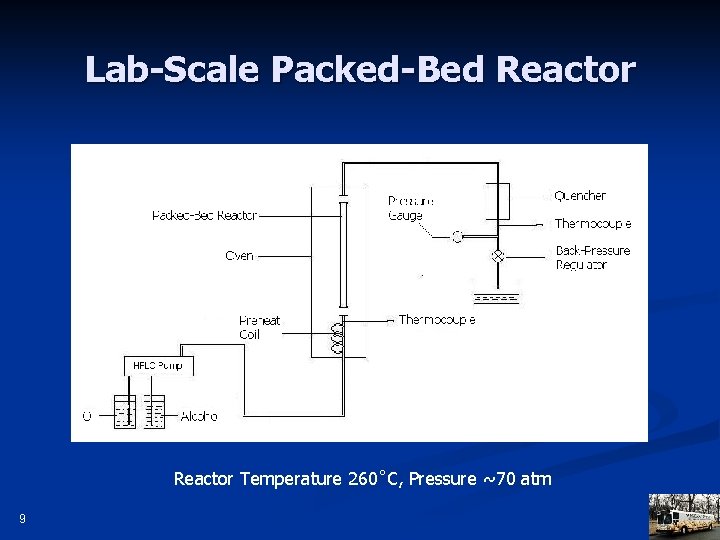
Lab-Scale Packed-Bed Reactor Temperature 260˚C, Pressure ~70 atm 9
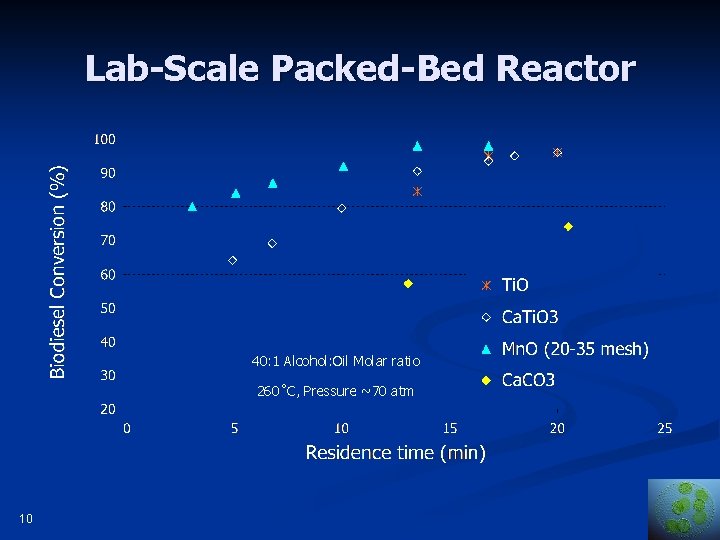
Lab-Scale Packed-Bed Reactor 40: 1 Alcohol: Oil Molar ratio 260˚C, Pressure ~70 atm 10
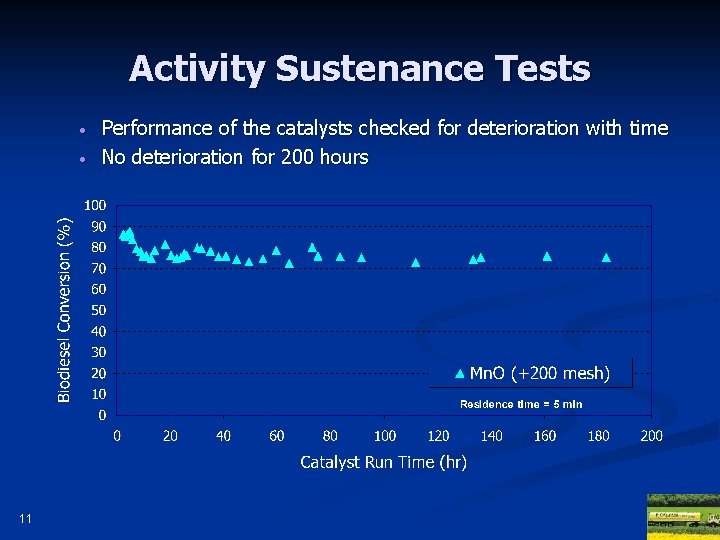
Activity Sustenance Tests • • 11 Performance of the catalysts checked for deterioration with time No deterioration for 200 hours
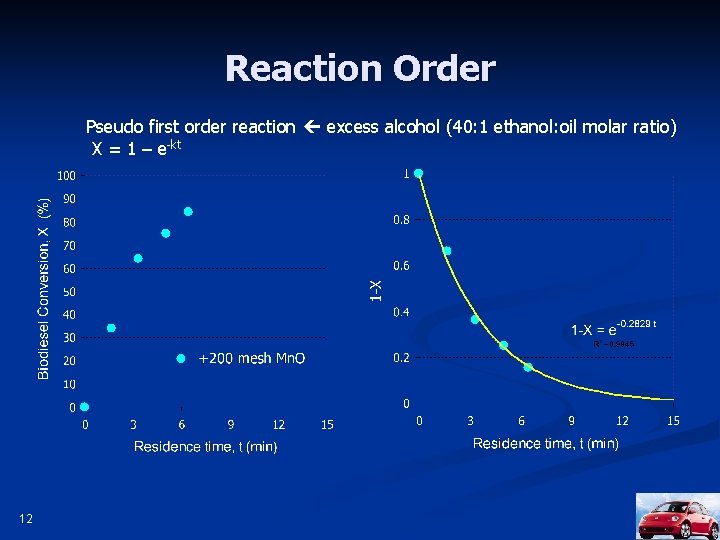
Reaction Order Pseudo first order reaction excess alcohol (40: 1 ethanol: oil molar ratio) X = 1 – e-kt 12
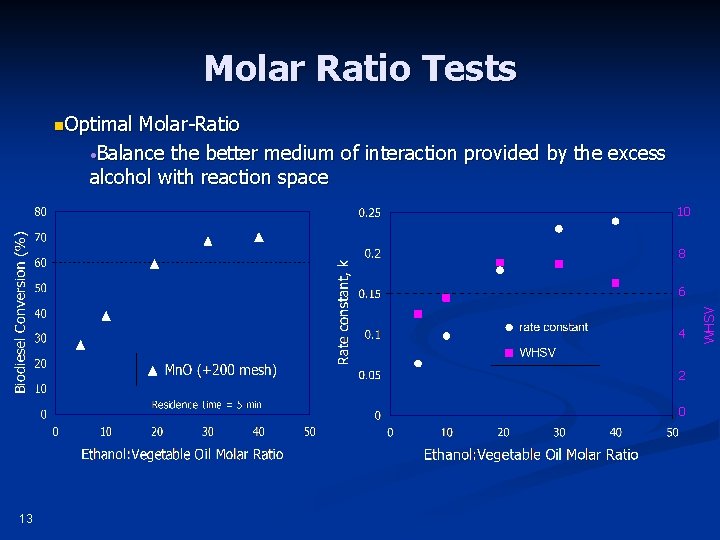
Molar Ratio Tests n. Optimal Molar-Ratio • Balance the better medium of interaction provided by the excess alcohol with reaction space 10 8 4 2 0 13 WHSV 6
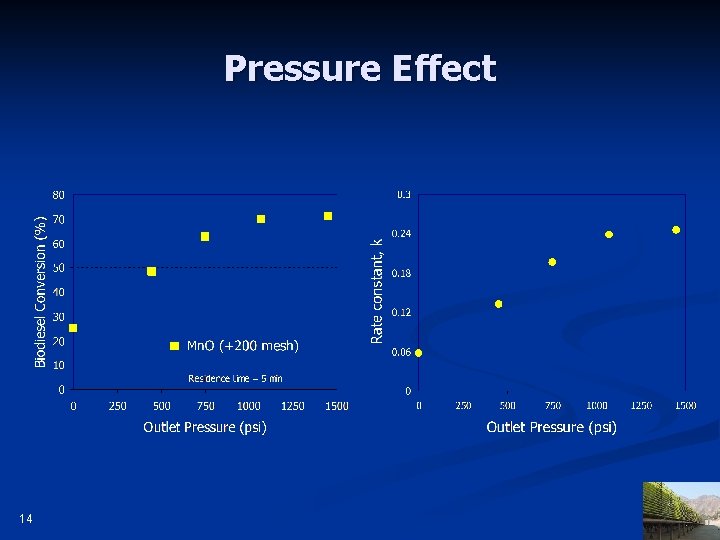
Pressure Effect 14
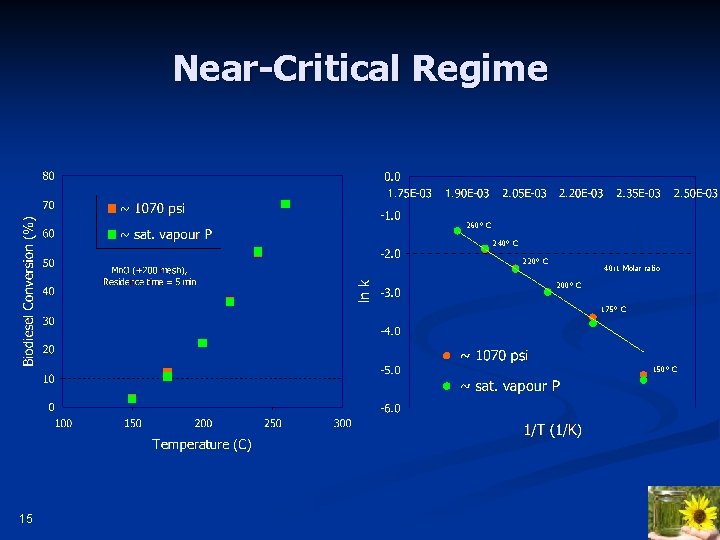
Near-Critical Regime 260° C 240° C 220° C 40: 1 Molar ratio 200° C 175° C 150° C 15

Related Studies 16 n Ni. O, VO, Fe. O, Zn. O and Co. O tested in packed-bed reactor. Only Fe. O gives similar results n Free fatty acid tested in packed-bed reactor show 97 % conversion in 7. 5 minutes – conversion rate even faster
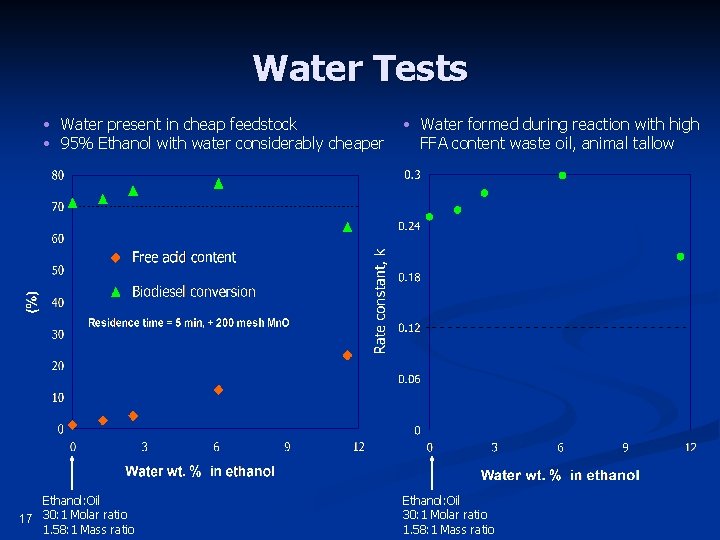
Water Tests • Water present in cheap feedstock • 95% Ethanol with water considerably cheaper Ethanol: Oil 17 30: 1 Molar ratio 1. 58: 1 Mass ratio • Water formed during reaction with high FFA content waste oil, animal tallow Ethanol: Oil 30: 1 Molar ratio 1. 58: 1 Mass ratio
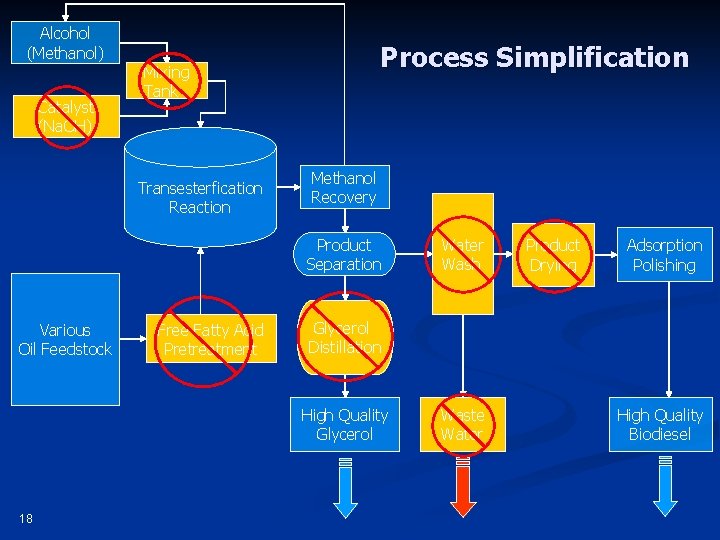
Alcohol (Methanol) Catalyst (Na. OH) Process Simplification Mixing Tank Transesterfication Reaction Methanol Recovery Product Separation Various Oil Feedstock Free Fatty Acid Pretreatment Product Drying Adsorption Polishing Glycerol Distillation High Quality Glycerol 18 Water Wash Waste Water High Quality Biodiesel
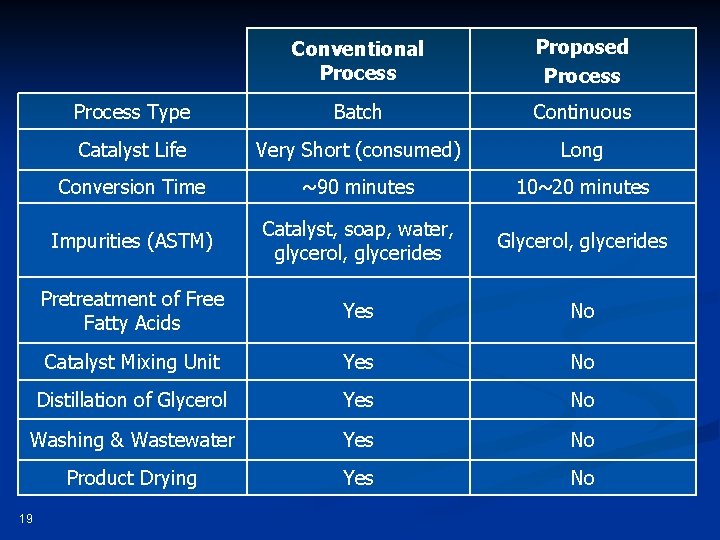
Conventional Process Proposed Process Type Batch Continuous Catalyst Life Very Short (consumed) Long Conversion Time ~90 minutes 10~20 minutes Impurities (ASTM) Catalyst, soap, water, glycerol, glycerides Glycerol, glycerides Pretreatment of Free Fatty Acids Yes No Catalyst Mixing Unit Yes No Distillation of Glycerol Yes No Washing & Wastewater Yes No Product Drying Yes No 19

Acknowledgements • • 20 Shaun Pardi Brian Dempsey Penn State Institutes of Energy and Environment Larry Duda and Ronald Danner Joe Perez and Wallis Lloyd CSPS Personnel – Adam Jones, Marc Russel, Ida Balashova, Lourdes Serna, Roman Galdamez Chris Torres and Brian Plunkett Restek

Thank You! Questions ? Our Team: Dheeban Kannan, Kevin Gombotz, Frank Higdon and Jack Matson
- Slides: 21