A New Test for Assessing the Risk of
















- Slides: 58

A New Test for Assessing the Risk of Ovarian Cancer in Women with Adnexal Mass Presenter Place Date C- 1

Ovarian Cancer is a Major Women's Health Problem • High morbidity and mortality • Appropriate treatment improves survival 1 – Oncology specialists – High volume centers • Need better risk assessment tools 1 ACOG C- 2 Practice Bulletin. Obstet Gynecol. 2007; 110: 201 -213.

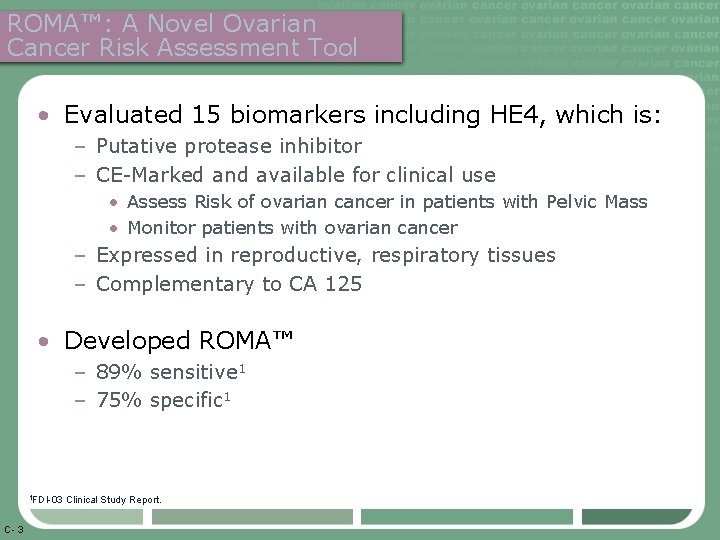
ROMA™: A Novel Ovarian Cancer Risk Assessment Tool • Evaluated 15 biomarkers including HE 4, which is: – Putative protease inhibitor – CE-Marked and available for clinical use • Assess Risk of ovarian cancer in patients with Pelvic Mass • Monitor patients with ovarian cancer – Expressed in reproductive, respiratory tissues – Complementary to CA 125 • Developed ROMA™ – 89% sensitive 1 – 75% specific 1 1 FDI-03 C- 3 Clinical Study Report.

ROMA™: A Novel Ovarian Cancer Risk Assessment Tool • Stratify risk of ovarian cancer • Ensure treatment by right surgeon/right facility • Used in conjunction with other Dx methods • Not intended for detection or screening C- 4

ROMA™ Will Improve Treatment of Women with Adnexal Mass C- 5

Agenda • Ovarian Cancer Risk Assessment • ROMA™ Development • Multicenter Validation Trial • Conclusion and Summary C- 6

Ovarian Cancer Risk Assessment C- 7

Need New Tools to Better Assess Ovarian Cancer Risk C- 8

Ovarian Cancer is a Deadly Disease • 204, 499 new cases in 2008 • 124, 860 deaths • Leading cause of gynecologic cancer deaths • 5 th leading cause of cancer deaths in women International Agency for Research on Cancer. Globocan 2002. http: //www-dep. iarc. fr/ C- 9

1 in 5 Women will have a Pelvic Mass • 20% of women will be diagnosed with an adnexal mass 1 • 5 - 10% of women will have surgery for an ovarian neoplasm (100, 000 to 200, 000)2 • 13 - 21% of these masses will be malignant 2 1 Curtin 2 NIH C- 10 JP. Gynecol Oncol. 1994; 55: S 42 -S 46. Consensus Development Conference Statement. Gynecol Oncol. 1994; 55: S 4 -S 14.

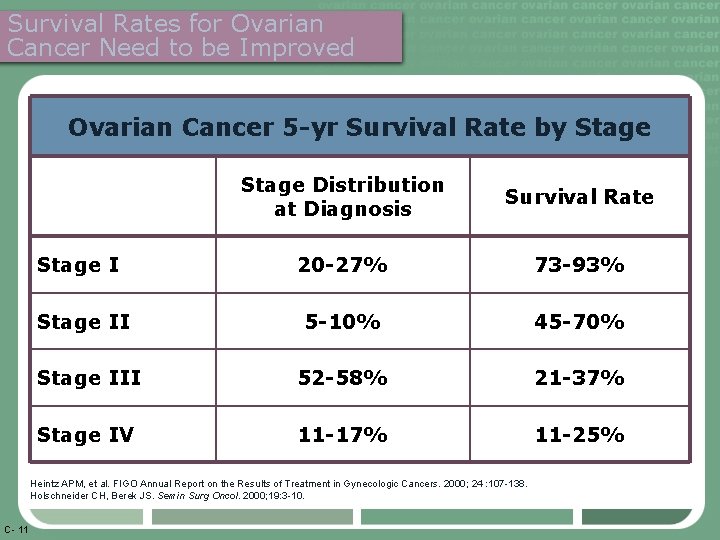
Survival Rates for Ovarian Cancer Need to be Improved Ovarian Cancer 5 -yr Survival Rate by Stage Distribution at Diagnosis Survival Rate Stage I 20 -27% 73 -93% Stage II 5 -10% 45 -70% Stage III 52 -58% 21 -37% Stage IV 11 -17% 11 -25% Heintz APM, et al. FIGO Annual Report on the Results of Treatment in Gynecologic Cancers. 2000; 24 : 107 -138. Holschneider CH, Berek JS. Semin Surg Oncol. 2000; 19: 3 -10. C- 11

How can we Affect Ovarian Cancer Survival? • Prevention • Screening • Early detection • Surgery • Chemotherapeutic agents C- 12

Surgery can Impact Survival • Cytoxan to Paclitaxel – 14 month survival advantage 1 • Intravenous to Intraperitoneal – 16 month survival advantage 2 • Surgery by gynecologic oncologist – 12 month survival advantage 3, 4 1 Mc. Guire WP et al. NEJM. 1996; 334(1): 1 -6. DK et al. NEJM. 2006; 354(1): 34 -43. 3 Engelen MJA et al. Cancer. 2006; 106(3): 589 -598. 4 Bristow RE et al. J Clin Oncol. 2002; 20(5): 1248 -1259 2 Armstrong C- 13

The Optimal Care for Ovarian Cancer • Cytoreductive surgery with complete surgical staging • Rationale for surgical staging: – Define the extent of disease – Determine the need for adjuvant treatment – Provide prognosis – Outline a plan of care C- 14

Surgical Debulking Increases Survival for Ovarian Cancer Optimal surgical debulking can include: • Hysterectomy • Removal of ovaries • Bowel resection • Peritoneal stripping • Diaphragmatic stripping • Lymph node debulking C- 15

Gynecologic Oncologists are Ovarian Cancer Specialists • Gynecologic oncologist – Recognized sub-specialty in US • Residency in Obstetrics and Gynecology (4 yrs) • Fellowship in Gynecologic Oncology (3 -4 yrs) – Outside US Gynecologists with high oncology surgical volume • Experienced in: – Surgical care – Medical management – Chemotherapy – Natural history C- 16

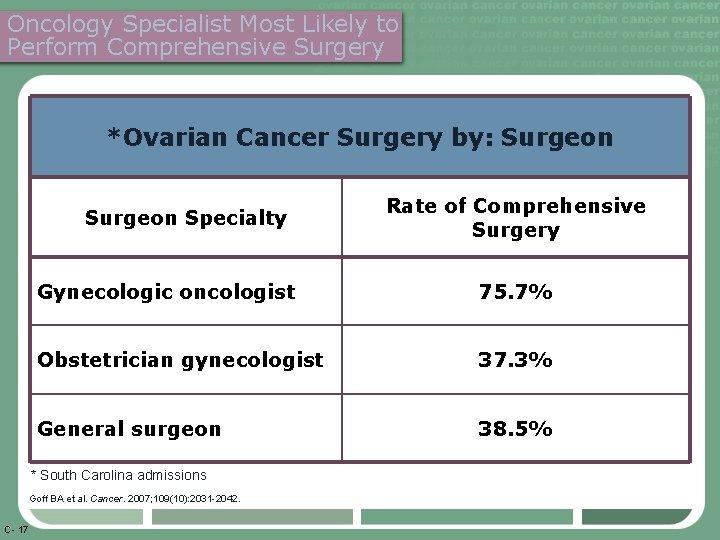
Oncology Specialist Most Likely to Perform Comprehensive Surgery *Ovarian Cancer Surgery by: Surgeon Specialty Gynecologic oncologist 75. 7% Obstetrician gynecologist 37. 3% General surgeon 38. 5% * South Carolina admissions Goff BA et al. Cancer. 2007; 109(10): 2031 -2042. C- 17 Rate of Comprehensive Surgery

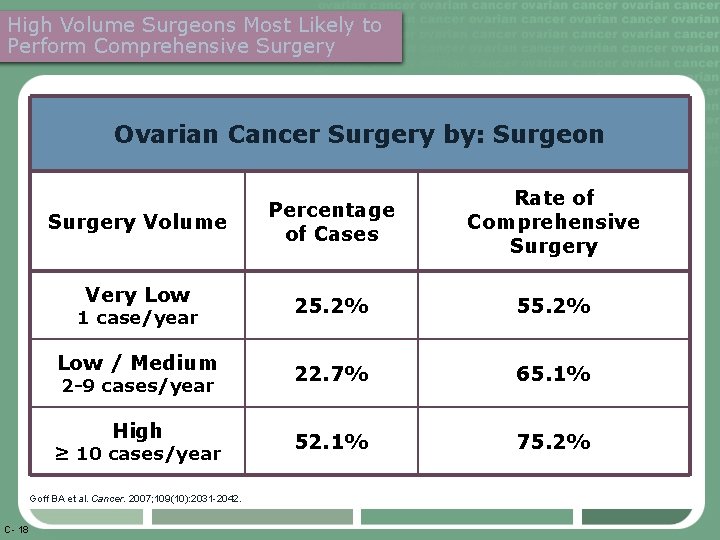
High Volume Surgeons Most Likely to Perform Comprehensive Surgery Ovarian Cancer Surgery by: Surgeon Surgery Volume Very Low 1 case/year Low / Medium 2 -9 cases/year High ≥ 10 cases/year Goff BA et al. Cancer. 2007; 109(10): 2031 -2042. C- 18 Percentage of Cases Rate of Comprehensive Surgery 25. 2% 55. 2% 22. 7% 65. 1% 52. 1% 75. 2%

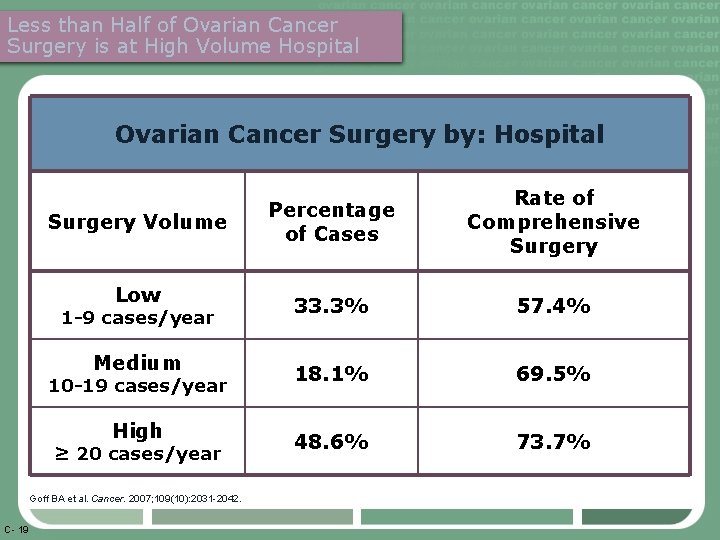
Less than Half of Ovarian Cancer Surgery is at High Volume Hospital Ovarian Cancer Surgery by: Hospital Surgery Volume Low 1 -9 cases/year Medium 10 -19 cases/year High ≥ 20 cases/year Goff BA et al. Cancer. 2007; 109(10): 2031 -2042. C- 19 Percentage of Cases Rate of Comprehensive Surgery 33. 3% 57. 4% 18. 1% 69. 5% 48. 6% 73. 7%

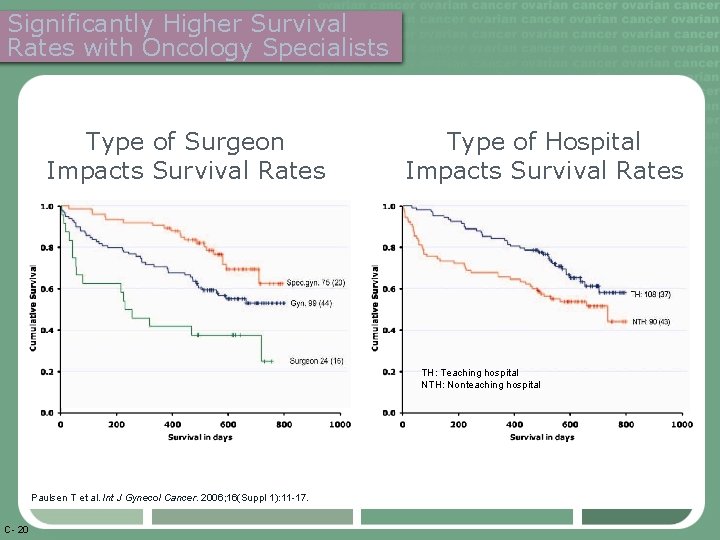
Significantly Higher Survival Rates with Oncology Specialists Type of Surgeon Impacts Survival Rates Type of Hospital Impacts Survival Rates TH: Teaching hospital NTH: Nonteaching hospital Paulsen T et al. Int J Gynecol Cancer. 2006; 16(Suppl 1): 11 -17. C- 20

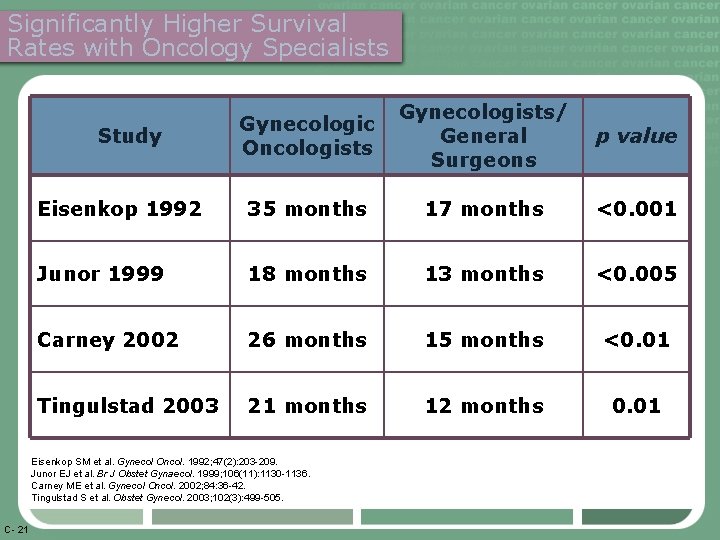
Significantly Higher Survival Rates with Oncology Specialists Gynecologic Oncologists Gynecologists/ General Surgeons p value Eisenkop 1992 35 months 17 months <0. 001 Junor 1999 18 months 13 months <0. 005 Carney 2002 26 months 15 months <0. 01 Tingulstad 2003 21 months 12 months 0. 01 Study Eisenkop SM et al. Gynecol Oncol. 1992; 47(2): 203 -209. Junor EJ et al. Br J Obstet Gynaecol. 1999; 106(11): 1130 -1136. Carney ME et al. Gynecol Oncol. 2002; 84: 36 -42. Tingulstad S et al. Obstet Gynecol. 2003; 102(3): 499 -505. C- 21

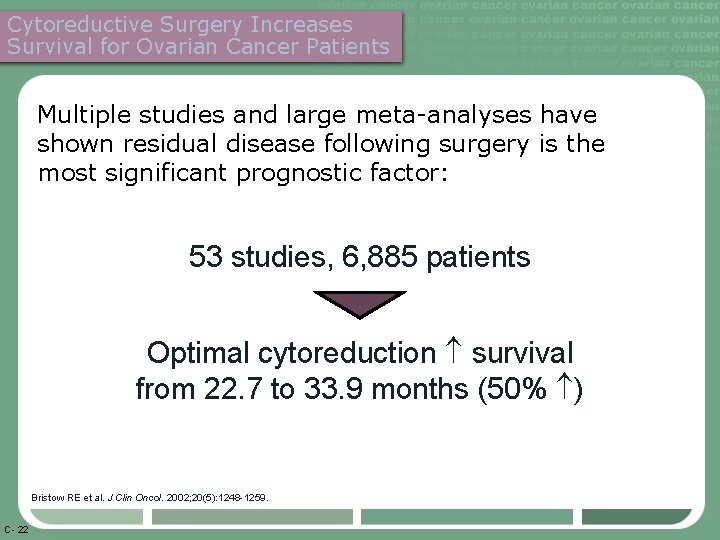
Cytoreductive Surgery Increases Survival for Ovarian Cancer Patients Multiple studies and large meta-analyses have shown residual disease following surgery is the most significant prognostic factor: 53 studies, 6, 885 patients Optimal cytoreduction survival from 22. 7 to 33. 9 months (50% ) Bristow RE et al. J Clin Oncol. 2002; 20(5): 1248 -1259. C- 22

Current Practice is Sub-Optimal for Ovarian Cancer Patients • In the US only 50% of women with ovarian cancer are operated on by high volume surgeons or at high volume centers 1 • Studies around the world show that survival rates are improved when patients have surgery by surgeons and at centers experienced in the management of ovarian cancer 2 1 Goff BA et al. Cancer. 2007; 109(10): 2031 -2042. Practice Bulletin. Obstet Gynecol. 2007; 110: 201 -213. 2 ACOG C- 23

Current Clinical Tools to Assess Risk of Ovarian Cancer • History • Physical exam • Imaging (US, CT and MRI) • Tumor markers (CA 125) C- 24

We can Improve the Care for Ovarian Cancer Patients • Better risk assessment • Improved patient care and management C- 25

Validation of ROMA™ as a Risk Assessment Tool and Patient Benefit C- 26

Development and Validation of ROMA™ • Two pilot studies combined to generate ROMA™ – Patients enrolled from: • Women and Infants’ Hospital, Providence RI • Massachusetts General Hospital, Boston MA • Pivotal trial (FDI-03) to validate ROMA™ – National trial – New patient cohort for validation C- 27

Primary Objective of Pivotal Trial • To validate a predictive model utilizing a dual marker assay of HE 4 and CA 125 to assess the risk for epithelial ovarian cancer including borderline/low malignant potential tumors in women with a pelvic mass FDI-03 Clinical Study Report. C- 28

Pivotal Trial Study Sites Chosen to Enrich Ovarian Cancer Population • 14 geographically dispersed sites across the US • Divisions of Gynecologic Oncology, within Departments of Obstetrics and Gynecology • Sites chosen to enrich study population FDI-03 Clinical Study Report. C- 29

Pivotal Trial Methods • Prospective double-blind multicenter trial • Eligibility criteria: – ≥ 18 years of age – Ovarian cyst or a pelvic mass – Planned surgical intervention • All EOC patients to be surgically staged • All blood samples obtained preoperatively • Central pathology review FDI-03 Clinical Study Report. C- 30

Pivotal Trial Enrollment • 566 patients enrolled • 530 evaluable patients – 246 premenopausal – 284 postmenopausal • 94% of patients were evaluable FDI-03 Clinical Study Report. C- 31

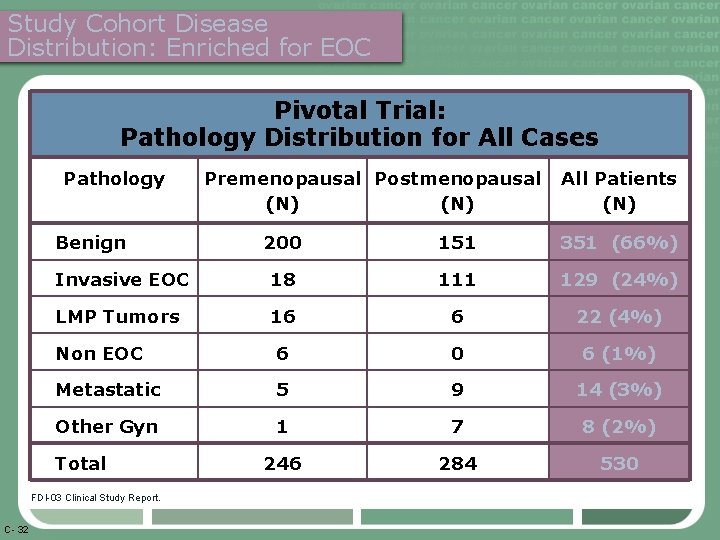
Study Cohort Disease Distribution: Enriched for EOC Pivotal Trial: Pathology Distribution for All Cases Pathology Benign All Patients (N) 200 151 351 (66%) Invasive EOC 18 111 129 (24%) LMP Tumors 16 6 22 (4%) Non EOC 6 0 6 (1%) Metastatic 5 9 14 (3%) Other Gyn 1 7 8 (2%) 246 284 530 Total FDI-03 Clinical Study Report. C- 32 Premenopausal Postmenopausal (N)

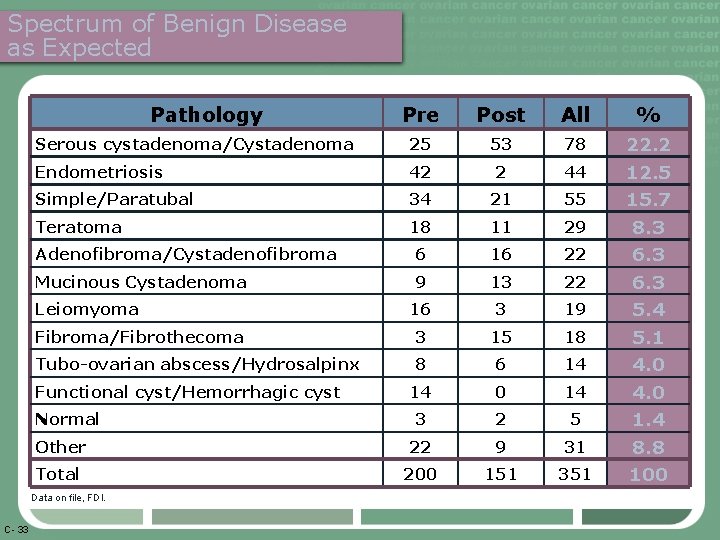
Spectrum of Benign Disease as Expected Pathology Pre Post All % Serous cystadenoma/Cystadenoma 25 53 78 22. 2 Endometriosis 42 2 44 12. 5 Simple/Paratubal 34 21 55 15. 7 Teratoma 18 11 29 8. 3 Adenofibroma/Cystadenofibroma 6 16 22 6. 3 Mucinous Cystadenoma 9 13 22 6. 3 16 3 19 5. 4 Fibroma/Fibrothecoma 3 15 18 5. 1 Tubo-ovarian abscess/Hydrosalpinx 8 6 14 4. 0 3 2 5 1. 4 Other 22 9 31 8. 8 Total 200 151 351 100 Leiomyoma Functional cyst/Hemorrhagic cyst Normal Data on file, FDI. C- 33

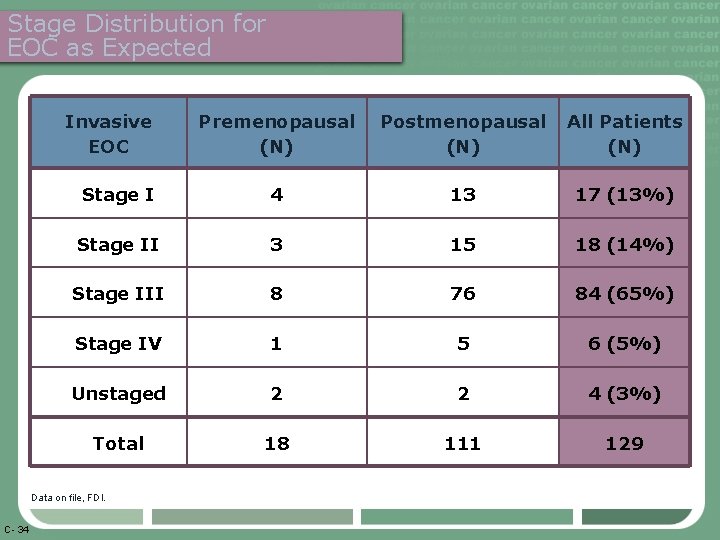
Stage Distribution for EOC as Expected Invasive EOC Premenopausal (N) Postmenopausal (N) All Patients (N) Stage I 4 13 17 (13%) Stage II 3 15 18 (14%) Stage III 8 76 84 (65%) Stage IV 1 5 6 (5%) Unstaged 2 2 4 (3%) Total 18 111 129 Data on file, FDI. C- 34

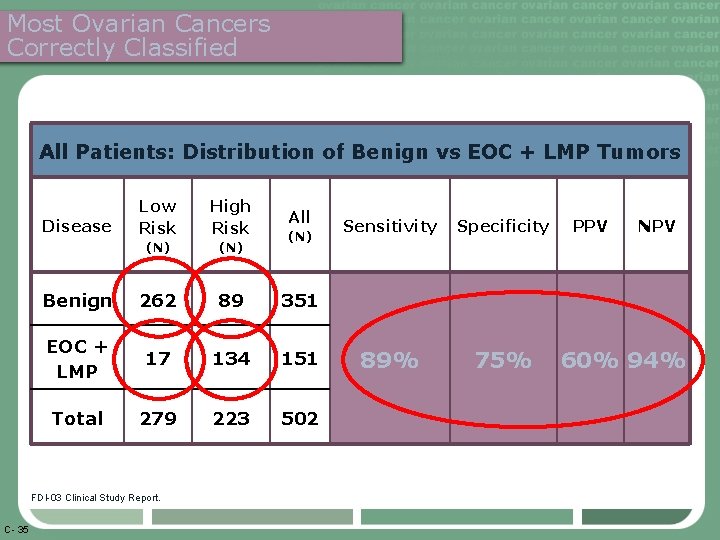
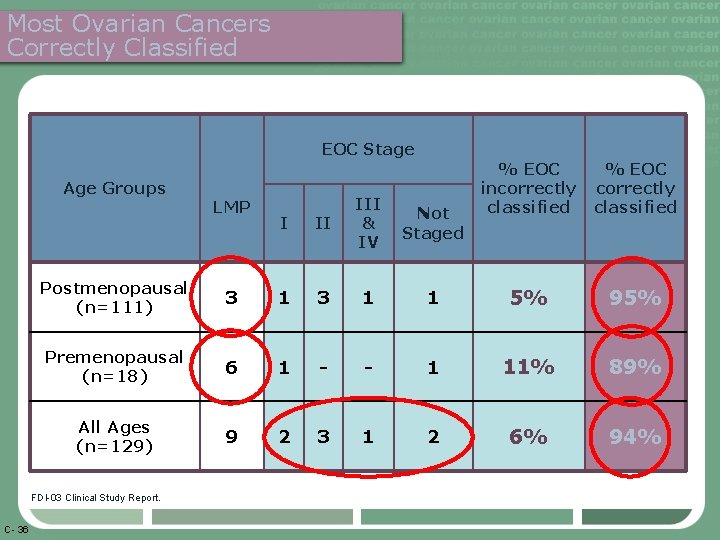
Most Ovarian Cancers Correctly Classified All Patients: Distribution of Benign vs EOC + LMP Tumors Low Risk High Risk (N) Benign 262 89 351 EOC + LMP 17 134 151 Total 279 223 502 Disease FDI-03 Clinical Study Report. C- 35 All (N) Sensitivity Specificity 89% 75% PPV NPV 60% 94%

Most Ovarian Cancers Correctly Classified EOC Stage Age Groups I II III & IV Not Staged % EOC correctly classified Postmenopausal (n=111) 3 1 1 5% 95% Premenopausal (n=18) 6 1 - - 1 11% 89% All Ages (n=129) 9 2 3 1 2 6% 94% FDI-03 Clinical Study Report. C- 36 LMP % EOC incorrectly classified

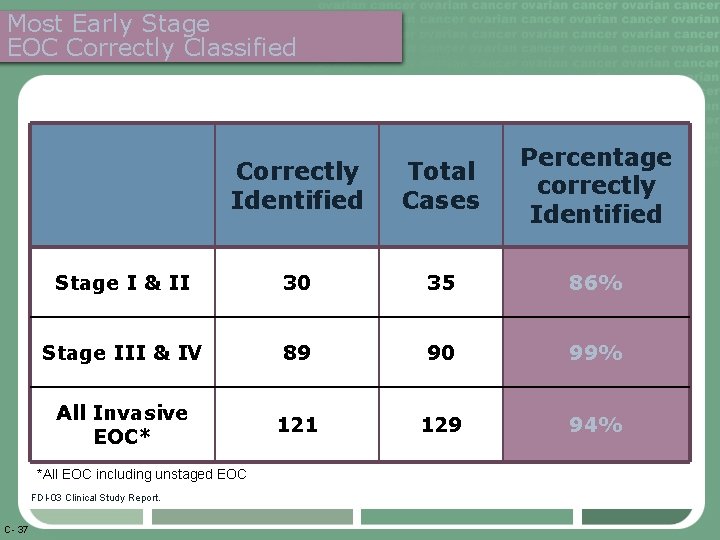
Most Early Stage EOC Correctly Classified Correctly Identified Total Cases Percentage correctly Identified Stage I & II 30 35 86% Stage III & IV 89 90 99% All Invasive EOC* 121 129 94% *All EOC including unstaged EOC FDI-03 Clinical Study Report. C- 37

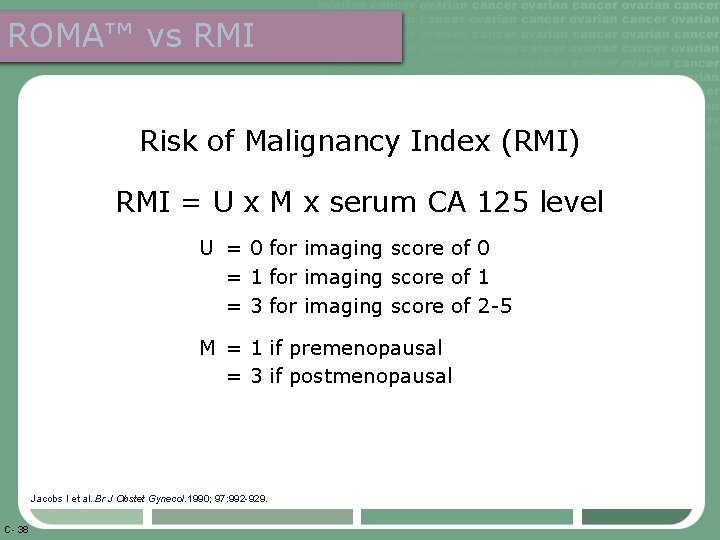
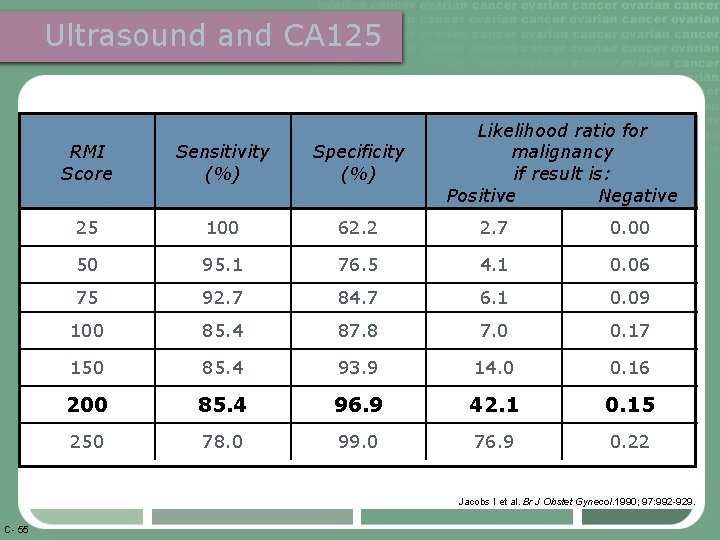
ROMA™ vs RMI Risk of Malignancy Index (RMI) RMI = U x M x serum CA 125 level U = 0 for imaging score of 0 = 1 for imaging score of 1 = 3 for imaging score of 2 -5 M = 1 if premenopausal = 3 if postmenopausal Jacobs I et al. Br J Obstet Gynecol. 1990; 97: 992 -929. C- 38

Secondary Analysis of ROMA™ vs RMI • Able to calculate an RMI for 80% of patients • Utilized US, CT scans and MRI results for RMI imaging scores C- 39

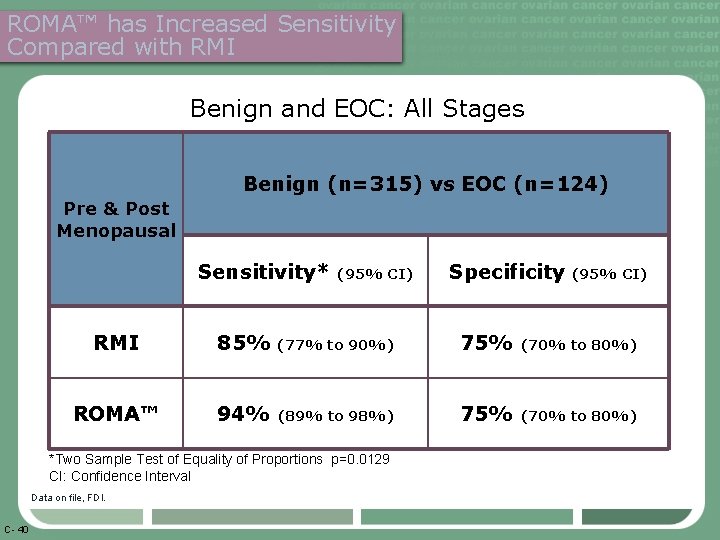
ROMA™ has Increased Sensitivity Compared with RMI Benign and EOC: All Stages Benign (n=315) vs EOC (n=124) Pre & Post Menopausal Sensitivity* (95% CI) RMI 85% (77% to 90%) 75% (70% to 80%) ROMA™ 94% (89% to 98%) 75% (70% to 80%) *Two Sample Test of Equality of Proportions p=0. 0129 CI: Confidence Interval Data on file, FDI. C- 40 Specificity

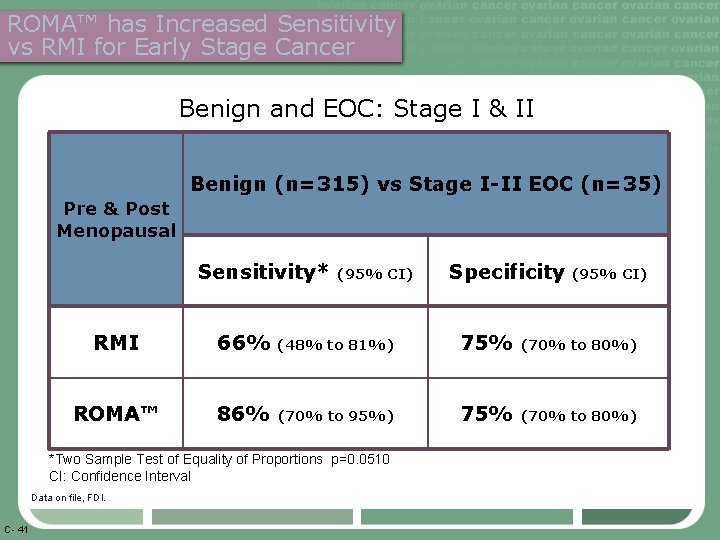
ROMA™ has Increased Sensitivity vs RMI for Early Stage Cancer Benign and EOC: Stage I & II Benign (n=315) vs Stage I-II EOC (n=35) Pre & Post Menopausal Sensitivity* (95% CI) RMI 66% (48% to 81%) 75% (70% to 80%) ROMA™ 86% (70% to 95%) 75% (70% to 80%) *Two Sample Test of Equality of Proportions p=0. 0510 CI: Confidence Interval Data on file, FDI. C- 41 Specificity

ROMA™ Demonstrates Superior Performance • Correctly identifies 94% of EOC 1 • Performs better than RMI • Simple and easy to use • Quantitative test • No subjective data • Assigns a risk for malignancy Data on file, FDI. C- 42

Additional Slides C- 43

Ovarian Cancer Epidemiology • Age adjusted incidence is 2 to 15 cases per 100, 000 women • Incidence rates are stable or slowly increasing C- 44

Surgical Staging • The current standard of care for ovarian cancer is cytoreductive surgery with complete surgical staging. • Complete surgical staging includes: – – – – – Laparotomy Hysterectomy Bilateral salpingo-oophorectomy Careful evaluation of all peritoneal surfaces Multiple washings for cytology Multiple peritoneal biopsies Hepatic and diaphragmatic cytology Omentectomy Pelvic and periaortic lymphadenectomy • Less than 50% of women undergoing surgery for an ovarian cancer will have an adequate staging or cytoreductive surgery 1, 2. Gynecologic Oncologists are trained in staging of ovarian cancer. 1 Carney 2 Mc. Gowan C- 45 ME et al. Gynecol Oncol. 2002; 84: 36 -42. L et al. Obstet Gynecol. 1985; 65(4): 568 -572.

Ovarian Cancer • Age at presentation is bimodal with peaks at age 40 and 60 years old • Symptoms often are nonspecific: – – – C- 46 Abdominal bloating Pelvic pressure GI symptoms Respiratory Constitutional

EDRN “Top Ten” Biomarkers for Detection of Ovarian Cancer • CA 125 • HE 4 • CA 15 -3 • CA 72 -4 • B 7 -H 4 (Ov 110) C- 47 • Transthyretin • IGFBP-2 • SMRP (Mesomark™) • HK 6 • Cytokeratin 19 (CYFRA 21 -1)

Biomarkers for Ovarian Cancer CA 125 • “Gold Standard” biomarker in ovarian cancer • Elevated CA 125 in 50% of Stage I disease and 80% of epithelial ovarian cancers 1 • Elevated in the pre-clinical asymptomatic phase of the disease Limitations – Elevated levels in benign gynecological disease 1, 2 – Low sensitivity in Stage I ovarian cancer – CA 125 alone is not a sensitive marker HE 4 • A commonly up-regulated biomarker in ovarian cancer • Serum HE 4 is a useful biomarker in the early diagnosis of ovarian cancer 1 NIH C- 48 Consensus Development Conference Statement. Gynecol Oncol. 1994; 55: S 4 -S 14. 2 ACOG Practice Bulletin. Obstet Gynecol. 2007; 110: 201 -213.

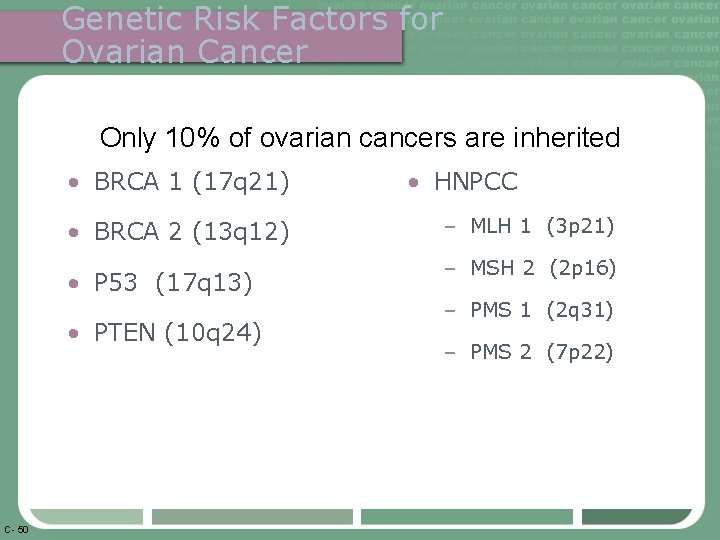
Genetic Risk Factors for Ovarian Cancer Only 10% of ovarian cancers are inherited • BRCA 1 (17 q 21) • BRCA 2 (13 q 12) • P 53 (17 q 13) • PTEN (10 q 24) C- 50 • HNPCC – MLH 1 (3 p 21) – MSH 2 (2 p 16) – PMS 1 (2 q 31) – PMS 2 (7 p 22)

Ultrasound Assessment of Pelvic Mass • Limitations of Ultrasound – Not all morphologic variables are commonly reported or measured – User variability (tertiary care vs community) – Ultrasound reporting is not standardized – Quality and complexity of machine (e. g. Doppler) – Complex algorithms Moore RG et al. J Clin Oncol. 2007; 25: 4159 -4161. C- 51

Preoperative Differentiation of Benign and Malignant Pelvic Masses • • To evaluate the risk of a malignancy To determine the need for surgery To triage patients To Improve the quality of care for patients – Allow patients to stay in their community – Appropriate patients referred to specialists • Medical-legal implications C- 52

Epidemiologic Risk Factors for Ovarian Cancer • Age • Early age at menarche • Late age at menopause • Nulliparity • Infertility • Caucasian race • History of endometriosis ACOG Practice Bulletin. Obstet Gynecol. 2007; 110: 201 -213. C- 53

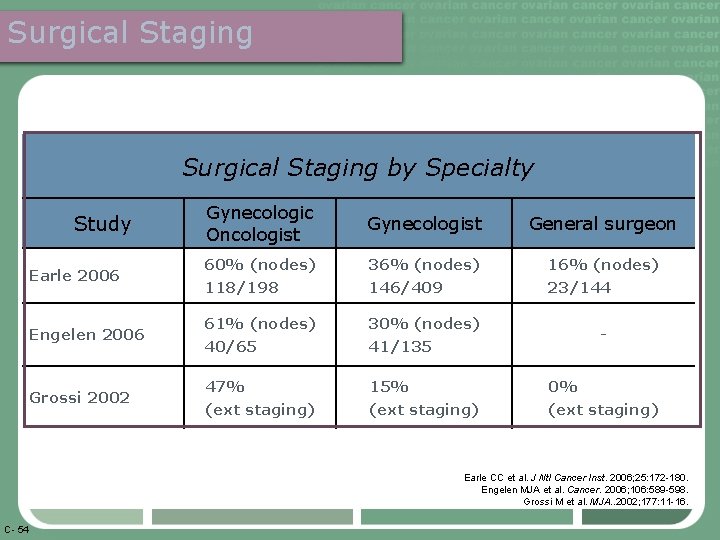
Surgical Staging by Specialty Study Earle 2006 Engelen 2006 Grossi 2002 Gynecologic Oncologist Gynecologist General surgeon 60% (nodes) 36% (nodes) 118/198 146/409 23/144 61% (nodes) 30% (nodes) 40/65 41/135 47% 15% 0% (ext staging) - Earle CC et al. J Ntl Cancer Inst. 2006; 25: 172 -180. Engelen MJA et al. Cancer. 2006; 106: 589 -598. Grossi M et al. MJA. . 2002; 177: 11 -16. C- 54

Ultrasound and CA 125 Likelihood ratio for malignancy if result is: Positive Negative RMI Score Sensitivity (%) Specificity (%) 25 100 62. 2 2. 7 0. 00 50 95. 1 76. 5 4. 1 0. 06 75 92. 7 84. 7 6. 1 0. 09 100 85. 4 87. 8 7. 0 0. 17 150 85. 4 93. 9 14. 0 0. 16 200 85. 4 96. 9 42. 1 0. 15 250 78. 0 99. 0 76. 9 0. 22 Jacobs I et al. Br J Obstet Gynecol. 1990; 97: 992 -929. C- 55

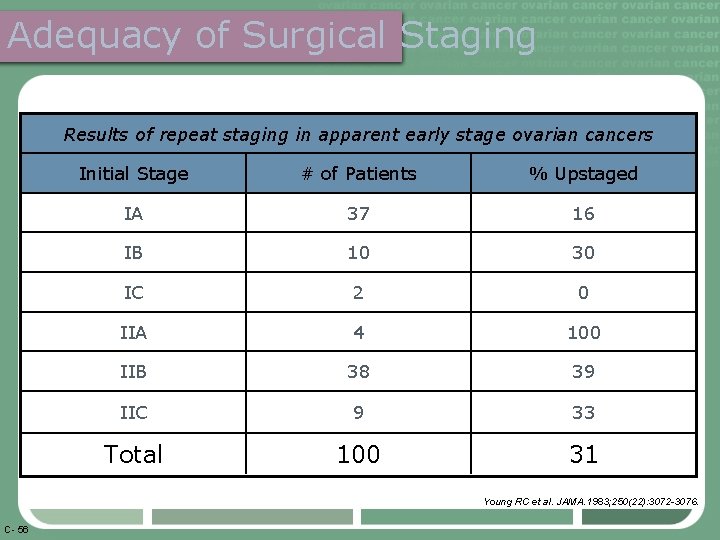
Adequacy of Surgical Staging Results of repeat staging in apparent early stage ovarian cancers Initial Stage # of Patients % Upstaged IA 37 16 IB 10 30 IC 2 0 IIA 4 100 IIB 38 39 IIC 9 33 Total 100 31 Young RC et al. JAMA. 1983; 250(22): 3072 -3076. C- 56

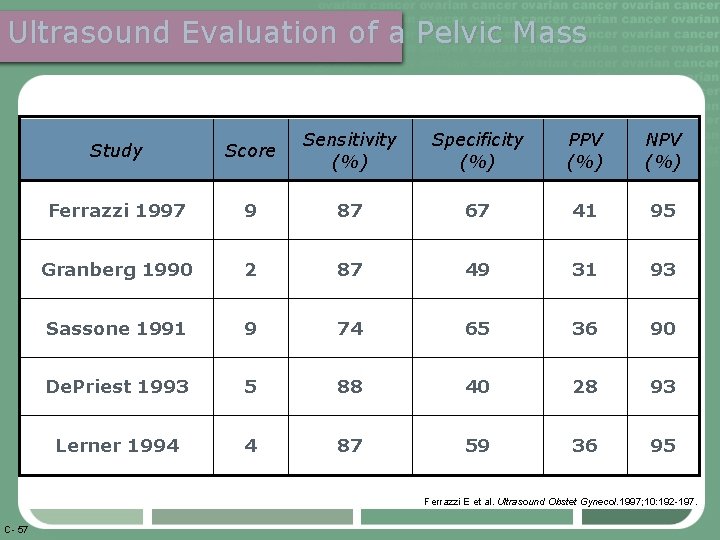
Ultrasound Evaluation of a Pelvic Mass Study Score Sensitivity (%) Specificity (%) PPV (%) NPV (%) Ferrazzi 1997 9 87 67 41 95 Granberg 1990 2 87 49 31 93 Sassone 1991 9 74 65 36 90 De. Priest 1993 5 88 40 28 93 Lerner 1994 4 87 59 36 95 Ferrazzi E et al. Ultrasound Obstet Gynecol. 1997; 10: 192 -197. C- 57

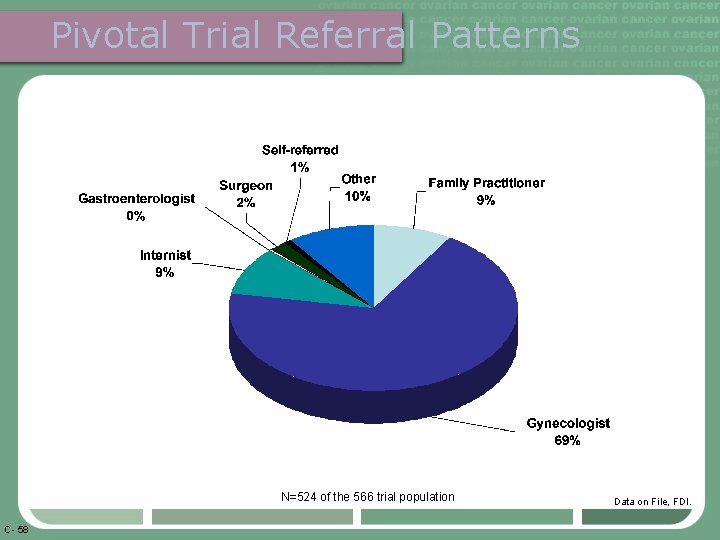
Pivotal Trial Referral Patterns N=524 of the 566 trial population C- 58 Data on File, FDI.

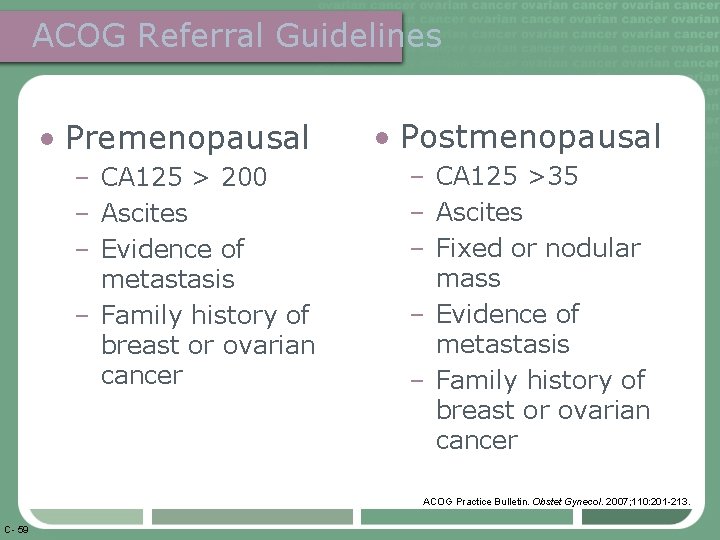
ACOG Referral Guidelines • Premenopausal – CA 125 > 200 – Ascites – Evidence of metastasis – Family history of breast or ovarian cancer • Postmenopausal – CA 125 >35 – Ascites – Fixed or nodular mass – Evidence of metastasis – Family history of breast or ovarian cancer ACOG Practice Bulletin. Obstet Gynecol. 2007; 110: 201 -213. C- 59