A New Disease Cluster Postural Orthostatic Tachycardia Syndrome






- Slides: 24

A New Disease Cluster: Postural Orthostatic Tachycardia Syndrome, Ehlers-Danlos Syndrome, and Mast Cell Activation Syndrome Ingrid Cheung, Juan Guzman, Roberto Mendoza-Londono, Scott Walsh, Karan Gandhi, Peter Vadas The ILC Ehlers Danlos & Chronic Pain Conference November 4, 2017

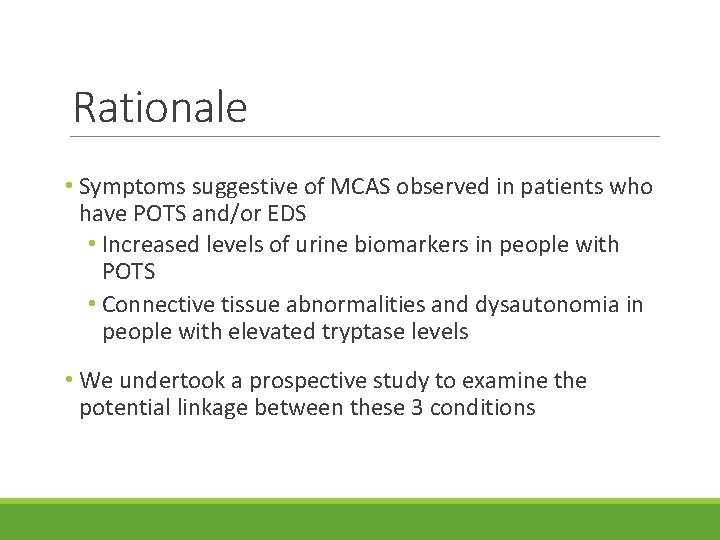
Rationale • Symptoms suggestive of MCAS observed in patients who have POTS and/or EDS • Increased levels of urine biomarkers in people with POTS • Connective tissue abnormalities and dysautonomia in people with elevated tryptase levels • We undertook a prospective study to examine the potential linkage between these 3 conditions

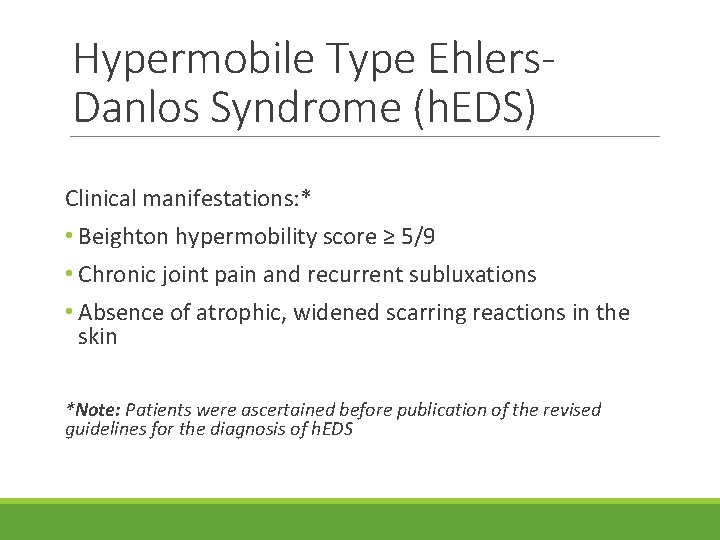
Hypermobile Type Ehlers. Danlos Syndrome (h. EDS) Clinical manifestations: * • Beighton hypermobility score ≥ 5/9 • Chronic joint pain and recurrent subluxations • Absence of atrophic, widened scarring reactions in the skin *Note: Patients were ascertained before publication of the revised guidelines for the diagnosis of h. EDS

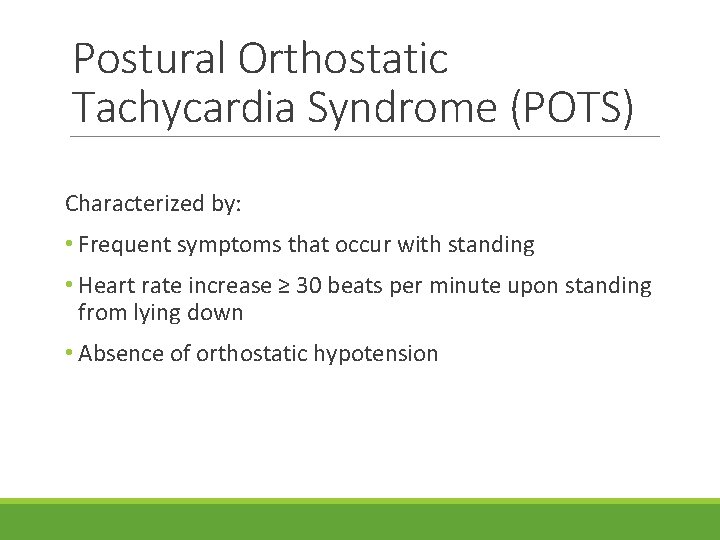
Postural Orthostatic Tachycardia Syndrome (POTS) Characterized by: • Frequent symptoms that occur with standing • Heart rate increase ≥ 30 beats per minute upon standing from lying down • Absence of orthostatic hypotension

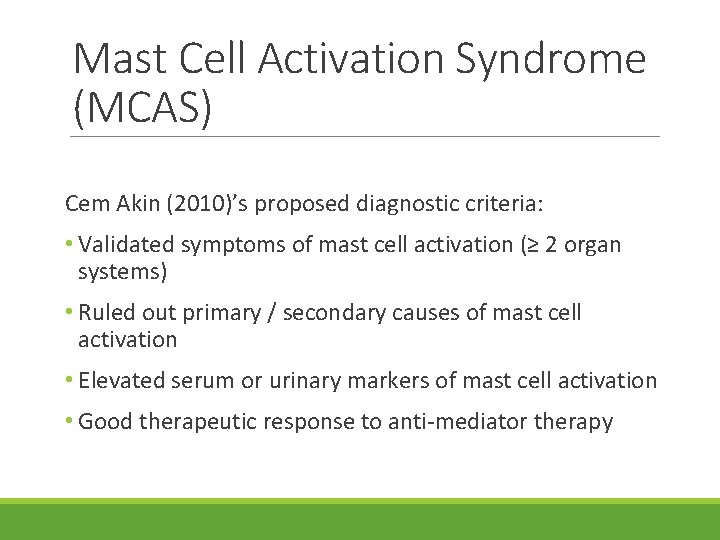
Mast Cell Activation Syndrome (MCAS) Cem Akin (2010)’s proposed diagnostic criteria: • Validated symptoms of mast cell activation (≥ 2 organ systems) • Ruled out primary / secondary causes of mast cell activation • Elevated serum or urinary markers of mast cell activation • Good therapeutic response to anti-mediator therapy

Phase I: Methods • Participants recruited from an online, North American patient support group • Recruitment criteria: • POTS: documented clinical diagnosis, confirmation via tilt-table test • h. EDS: documented diagnosis, Beighton hypermobility score ≥ 5/9 • MCAS: assessed presentation of validated symptoms of mast cell mediator release

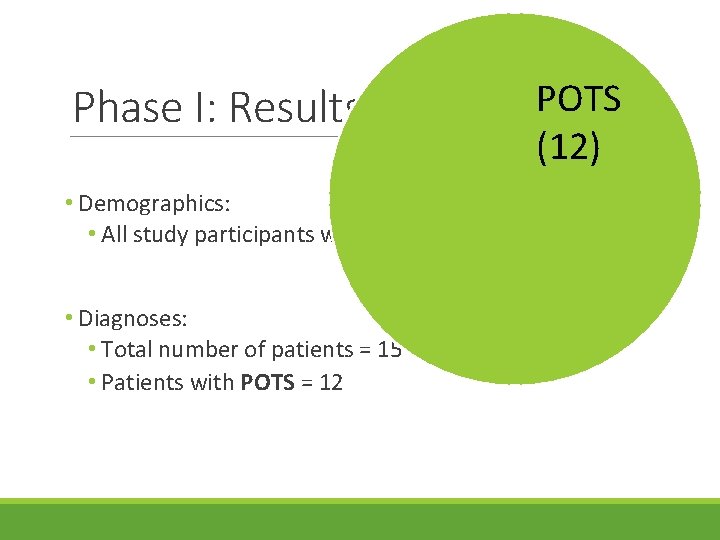
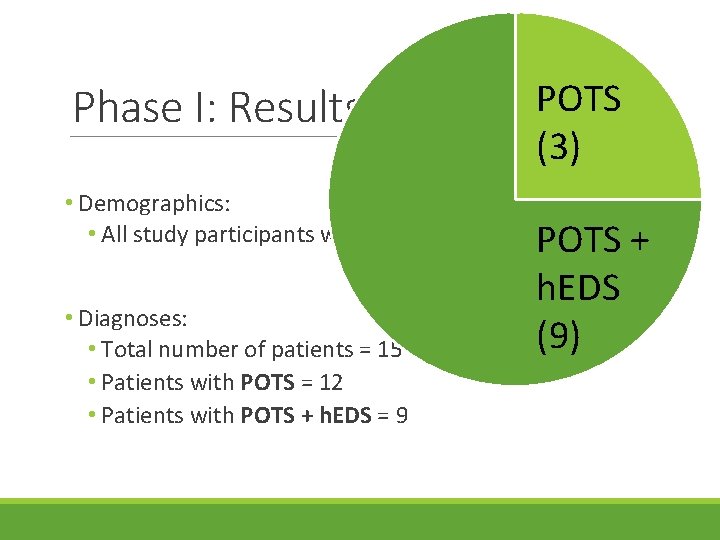
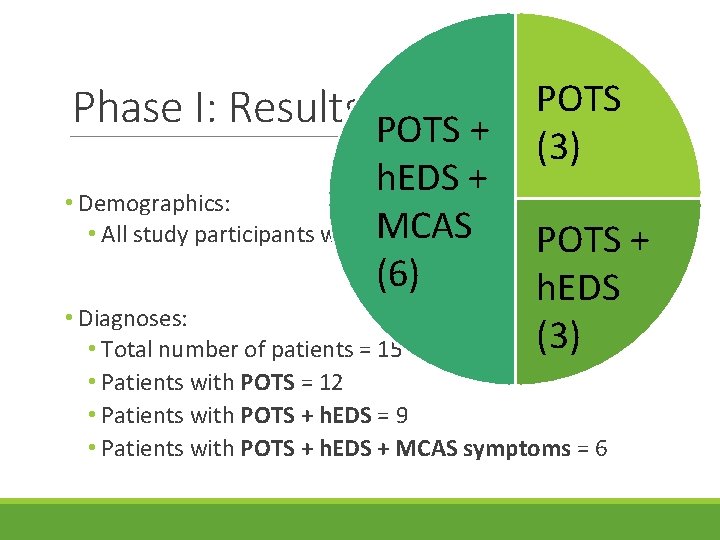
Phase I: Results • Demographics: • All study participants were female, >25 years old • Diagnoses: • Total number of patients = 15

Phase I: Results POTS (12) • Demographics: • All study participants were female, >25 years old • Diagnoses: • Total number of patients = 15 • Patients with POTS = 12

Phase I: Results POTS (3) • Demographics: • All study participants were female, >25 years old POTS • Diagnoses: • Total number of patients = 15 • Patients with POTS = 12 • Patients with POTS + h. EDS = 9 h. EDS (9) +

Phase I: Results POTS + POTS (3) h. EDS + • Demographics: • All study participants were MCAS female, >25 years old + POTS (6) h. EDS • Diagnoses: (3) • Total number of patients = 15 • Patients with POTS = 12 • Patients with POTS + h. EDS = 9 • Patients with POTS + h. EDS + MCAS symptoms = 6

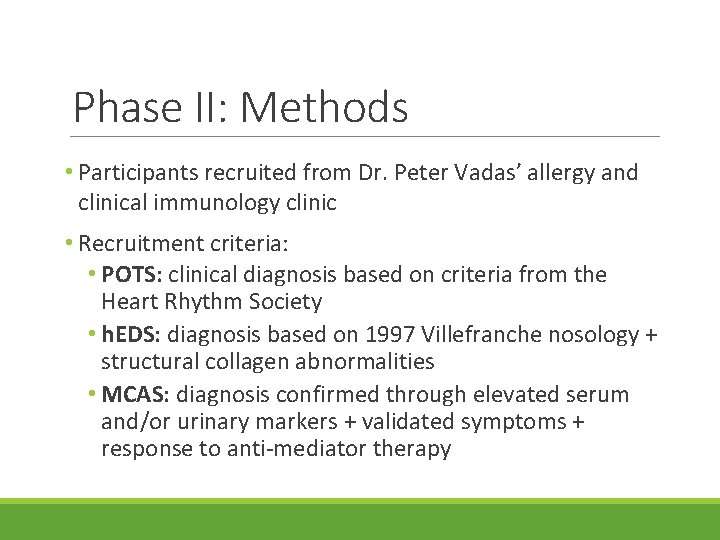
Phase II: Methods • Participants recruited from Dr. Vadas’ allergy and clinical immunology clinic • Recruitment criteria: • POTS: clinical diagnosis based on criteria from the Heart Rhythm Society • h. EDS: diagnosis based on 1997 Villefranche nosology + structural collagen abnormalities • MCAS: diagnosis confirmed through elevated serum and/or urinary markers + validated symptoms + response to anti-mediator therapy



Phase II: Methods • Participants recruited from Dr. Peter Vadas’ allergy and clinical immunology clinic • Recruitment criteria: • POTS: clinical diagnosis based on criteria from the Heart Rhythm Society • h. EDS: diagnosis based on 1997 Villefranche nosology + structural collagen abnormalities • MCAS: diagnosis confirmed through elevated serum and/or urinary markers + validated symptoms + response to anti-mediator therapy

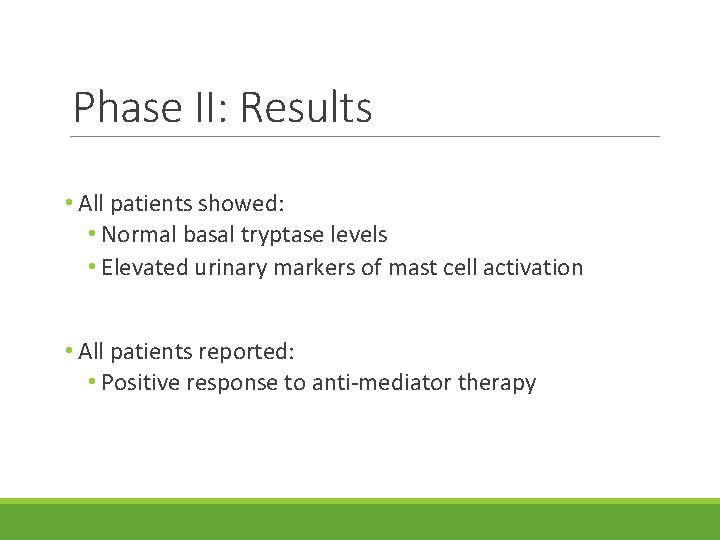
Phase II: Results • All patients showed: • Normal basal tryptase levels • Elevated urinary markers of mast cell activation • All patients reported: • Positive response to anti-mediator therapy

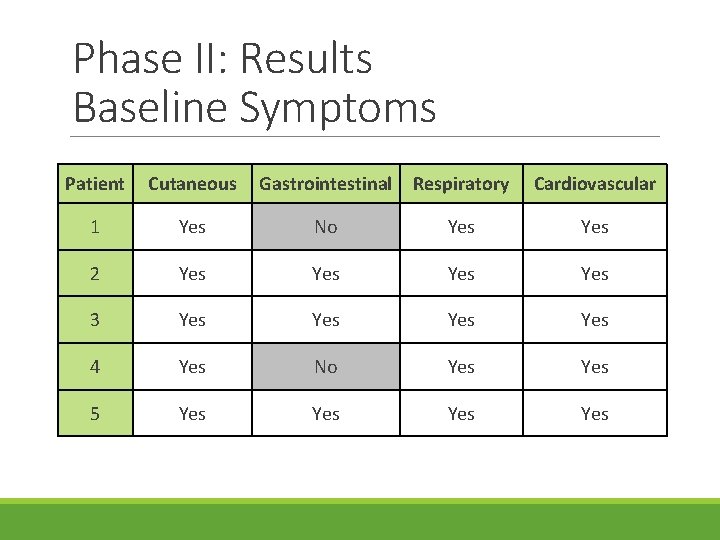
Phase II: Results Baseline Symptoms Patient Cutaneous Gastrointestinal Respiratory Cardiovascular 1 Yes No Yes 2 Yes Yes 3 Yes Yes 4 Yes No Yes 5 Yes Yes

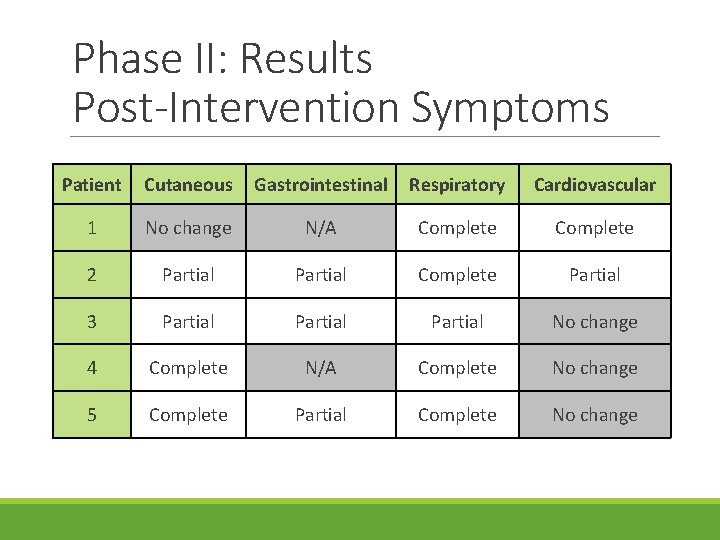
Phase II: Results Post-Intervention Symptoms Patient Cutaneous Gastrointestinal Respiratory Cardiovascular 1 No change N/A Complete 2 Partial Complete Partial 3 Partial No change 4 Complete N/A Complete No change 5 Complete Partial Complete No change

Discussion • There has been little work published in this area • Shibao et al. (2005) • Milner et al. (2014, 2016) • Afrin et al. (March 2017) • Our findings suggest that those with POTS and/or h. EDS have a higher risk of also having a diagnosis of MCAS • Anti-mediator therapy may be an effective treatment to control MCAS-associated symptoms in these patients

Study Conclusions • There is a lot of overlap between symptoms of h. EDS, POTS, and MCAS • Therapies must be directed at each of these 3 diagnoses individually in order to optimize treatment and quality of life

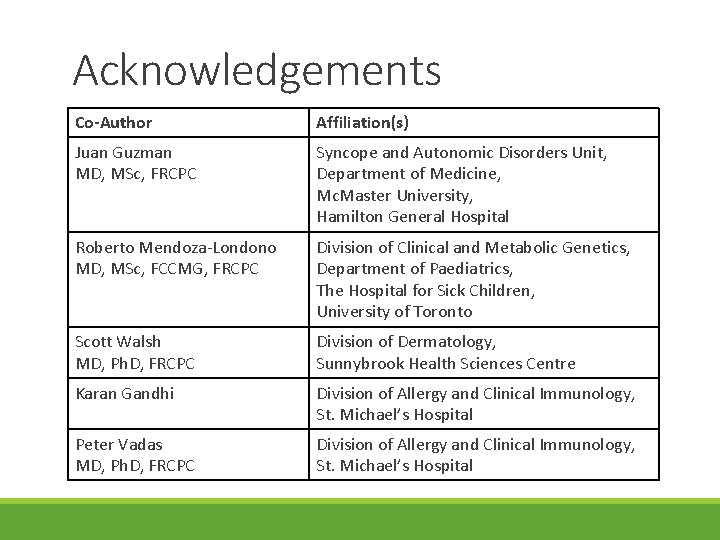
Acknowledgements Co-Author Affiliation(s) Juan Guzman MD, MSc, FRCPC Syncope and Autonomic Disorders Unit, Department of Medicine, Mc. Master University, Hamilton General Hospital Roberto Mendoza-Londono MD, MSc, FCCMG, FRCPC Division of Clinical and Metabolic Genetics, Department of Paediatrics, The Hospital for Sick Children, University of Toronto Scott Walsh MD, Ph. D, FRCPC Division of Dermatology, Sunnybrook Health Sciences Centre Karan Gandhi Division of Allergy and Clinical Immunology, St. Michael’s Hospital Peter Vadas MD, Ph. D, FRCPC Division of Allergy and Clinical Immunology, St. Michael’s Hospital

Next Steps • Accrue more patients for this study • Formal quality of life assessments to assess affect of mast cellmediated therapy on patients’ quality of life • Molecular studies to investigate mechanism that causes mast cell activation in patients with h. EDS and POTS

Limitations • Revised h. EDS nosology • More narrow definition • Small sample size in both study phases • Lack of statistical power • Limits generalizability • Selection bias • Phase I: self-identified patients • Phase II: recruitment from a single, specialized, allergy / immunology practice • Recall bias • Patient self-reported symptoms • Placebo effect / Co-therapies • Concurrent treatment for POTS and/or h. EDS diagnoses

MCAS: Diagnostic Criteria 1. Multiple mast cell mediator-induced symptoms, affecting ≥ 2 of the following organ systems: Dermatologic: itching, flushing, urticaria Gastrointestinal: crampy abdominal pain, nausea/vomiting, diarrhea Cardiovascular: tachycardia, labile blood pressure, syncope/pre-syncope Respiratory: wheezing, cough, chest tightness, dyspnea Naso-ocular: conjunctival injection, severely itchy eyes, nasal congestion 2. Rule out primary and secondary causes of mast cell activation, such as: Primary: systemic mastocytosis, mast cell clonality Secondary: allergy or other underlying mast cell activation trigger 3. Evidence of abnormal levels of validated urinary or serum markers of mast cell activation, including: Absence of elevated total serum tryptase (<11. 4 ng/m. L) Increase in the following less specific markers: 24 -hour urine histamine metabolites, prostaglandin D 2, 11 -β-prostaglandin F 2α 4. Decreased frequency or severity of mast cell activation-related symptoms, in response to antimast cell mediator therapy, such as: H 1 - and H 2 -histamine receptor antagonists: diphenhydramine, loratadine, ranitidine Anti-leukotriene blockers: montelukast, zafirlukast Mast cell stabilizers: cromolyn sodium, ketotifen

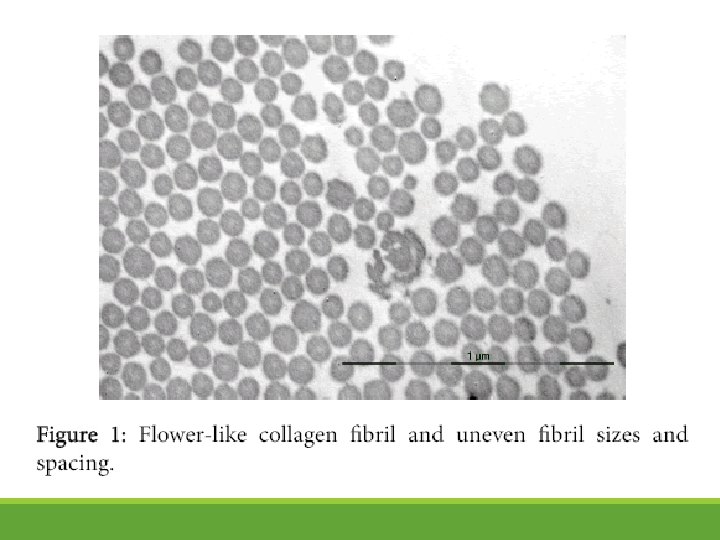
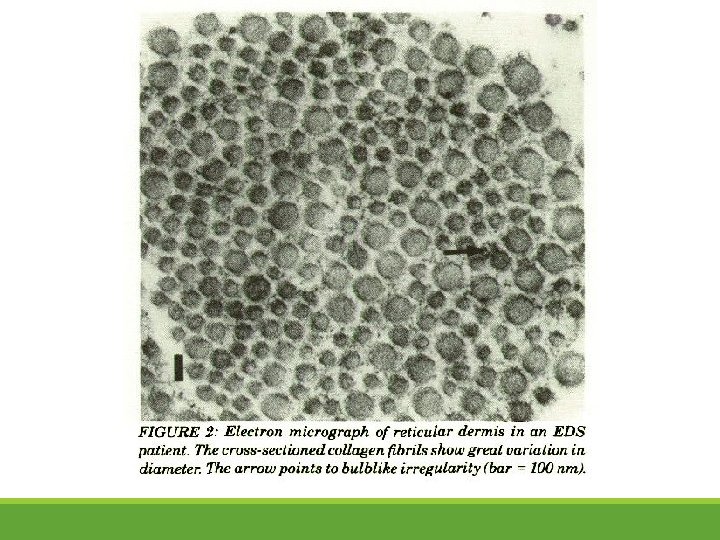
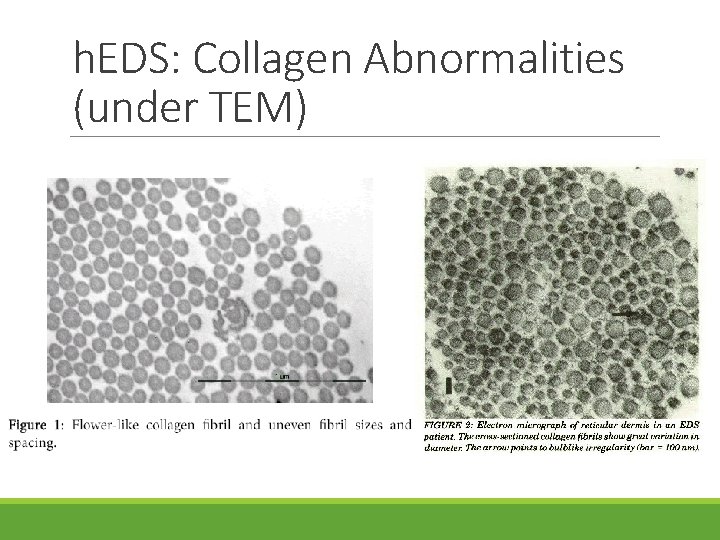
h. EDS: Collagen Abnormalities (under TEM)