A method for measuring phosphorus nutrient limitation using





- Slides: 12

A method for measuring phosphorus nutrient limitation using the oxygen isotopic composition of phosphates Joseph Murray Arizona State University Arizona Space Grant Symposium April 19 th, 2008 Acknowledgements: Dr. Ariel Anbar Dr. Achim Herrmann Dr. James Elser

§ The goal of this project is to develop a new method for detecting nutrient limitation in natural settings § The project is exploring the use of oxygen isotopic signatures in phosphates as a way of measuring phosphorus limitation § Initial research will be on application in freshwater ecology, but future applications have exciting potential

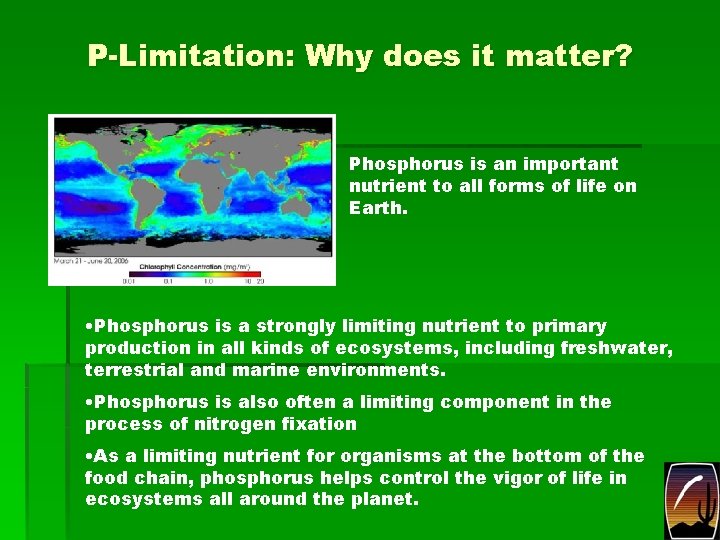
P-Limitation: Why does it matter? Phosphorus is an important nutrient to all forms of life on Earth. • Phosphorus is a strongly limiting nutrient to primary production in all kinds of ecosystems, including freshwater, terrestrial and marine environments. • Phosphorus is also often a limiting component in the process of nitrogen fixation • As a limiting nutrient for organisms at the bottom of the food chain, phosphorus helps control the vigor of life in ecosystems all around the planet.

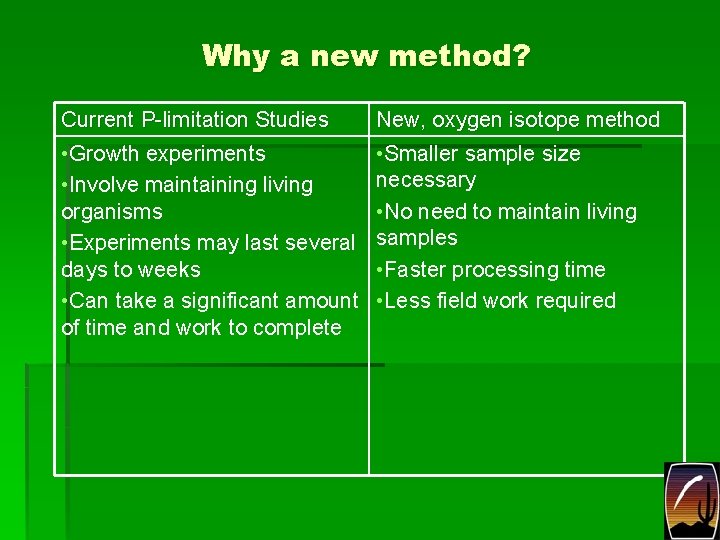
Why a new method? Current P-limitation Studies • Growth experiments • Involve maintaining living organisms • Experiments may last several days to weeks • Can take a significant amount of time and work to complete New, oxygen isotope method • Smaller sample size necessary • No need to maintain living samples • Faster processing time • Less field work required

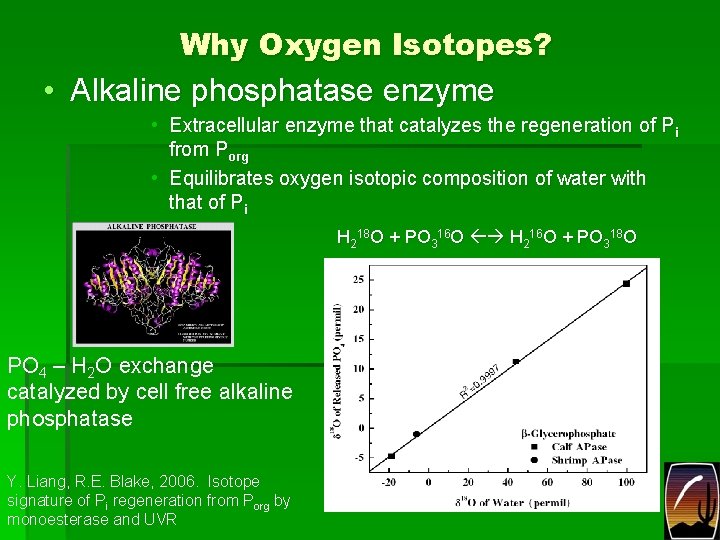
Why Oxygen Isotopes? • Alkaline phosphatase enzyme • Extracellular enzyme that catalyzes the regeneration of Pi from Porg • Equilibrates oxygen isotopic composition of water with that of Pi H 218 O + PO 316 O H 216 O + PO 318 O PO 4 – H 2 O exchange catalyzed by cell free alkaline phosphatase Y. Liang, R. E. Blake, 2006. Isotope signature of Pi regeneration from Porg by monoesterase and UVR

• Organisms often increase production of alkaline phosphatase under P-limiting conditions • Expected oxygen isotopic composition differences: • P-Limited environment Ø Higher concentration of extracellular alkaline phosphatase Ø High Pi turnover rate Ø δ 18 OP should be equilibrated with δ 18 OW • P-Sufficient environment Ø Lower concentration of extracellular alkaline phosphatase Ø Low Pi turnover rate Ø Would not expect to see δ 18 OP - δ 18 OW equilibration • Plan: measure phosphate oxygen isotopic composition of P-limited and P-sufficient organisms to check for this proposed isotopic difference

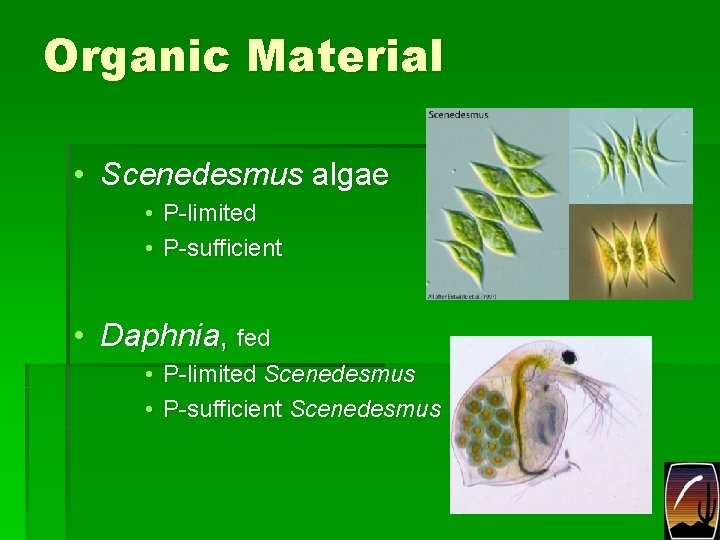
Organic Material • Scenedesmus algae • P-limited • P-sufficient • Daphnia, fed • P-limited Scenedesmus • P-sufficient Scenedesmus

Phosphate extraction and purification Indicates procedural steps requiring more investigation Digestion of organic material by UV radiation HNO 3 Precipitation of cerium phosphate using cerium nitrate. Centrifuge. HNO 3 Ion Exchange Chromatography: AG-50 x 8 cation exchange resin. . 20 M Ag. NO 3. 35 M NH 4 NO 3. 74 M NH 4 OH Ultraviolet Radiation Lamp To TC/EA and mass spectrometer Next Slide Precipitate as silver phosphate using Silver Amine solution

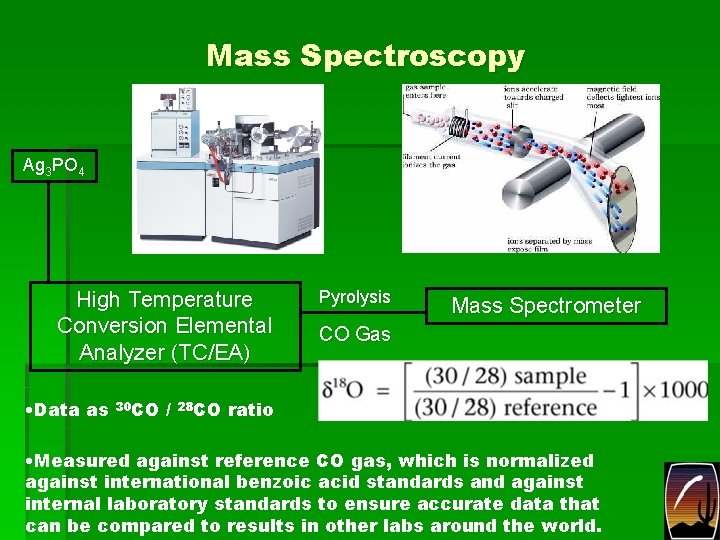
Mass Spectroscopy Ag 3 PO 4 High Temperature Conversion Elemental Analyzer (TC/EA) • Data as 30 CO / 28 CO Pyrolysis Mass Spectrometer CO Gas ratio • Measured against reference CO gas, which is normalized against international benzoic acid standards and against internal laboratory standards to ensure accurate data that can be compared to results in other labs around the world.

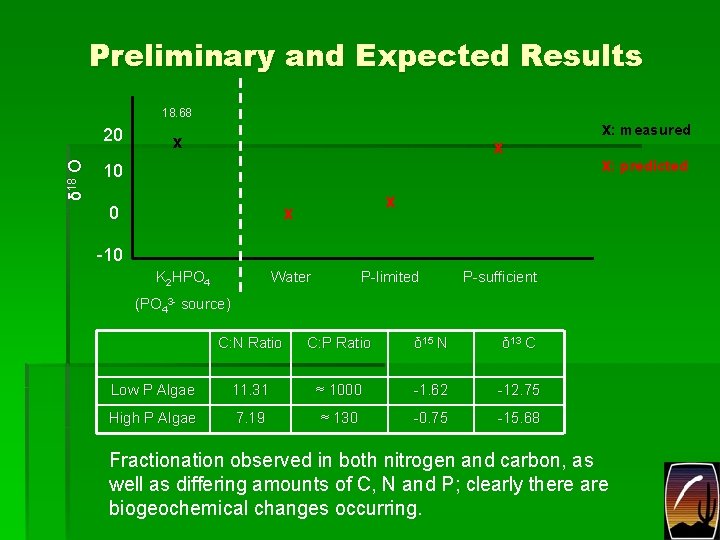
Preliminary and Expected Results 18. 68 δ 18 O 20 x x 10 0 x: measured x: predicted x x -10 K 2 HPO 4 Water P-limited P-sufficient (PO 43 - source) C: N Ratio C: P Ratio δ 15 N δ 13 C Low P Algae 11. 31 ≈ 1000 -1. 62 -12. 75 High P Algae 7. 19 ≈ 130 -0. 75 -15. 68 Fractionation observed in both nitrogen and carbon, as well as differing amounts of C, N and P; clearly there are biogeochemical changes occurring.

Possible Future Implications § Current P-limitation studies § Analysis of the ecological effects of anthropogenic phosphorus pollution during the past century § Reconstruction of nutrient history throughout longer periods of time

Thank You to. . . § ASU/NASA Space Grant Program § Dr. Achim Herrmann of Barrett, the Honors College § Dr. Ariel Anbar and Dr. Gwyneth Gordon of the W. M. Keck Foundation Laboratory for Environmental Biogeochemistry § Dr. James Elser and Marcia Kyle of the ASU School of Life Sciences § ASU School of Life Sciences Undergraduate Research Program THE END