A lowfrequency ultrasound reactor for continuous flow for







- Slides: 19

A low-frequency ultrasound reactor for continuous flow for precipitation reactions Claire Delacour, Zhengya Dong, Simon Kuhn Department of Chemical Engineering, KU Leuven COSMIC Project – H 2020 23/10/2018 International Conference on Micro Technology – IMRET 2018

Objectives COSMIC Project: European Training Network for Continuous Sonication and Microwave Reactors → Marie Sklodowska-Curie actions Aim of my Ph. D: Design of ultrasound integrated micro-reactors for clogging prevention Aim of this presentation: ultrasonic microreactor for precipitation and temperature control 2

Outline Objectives Background Materials and methods Results and discussions Conclusion USµR 3

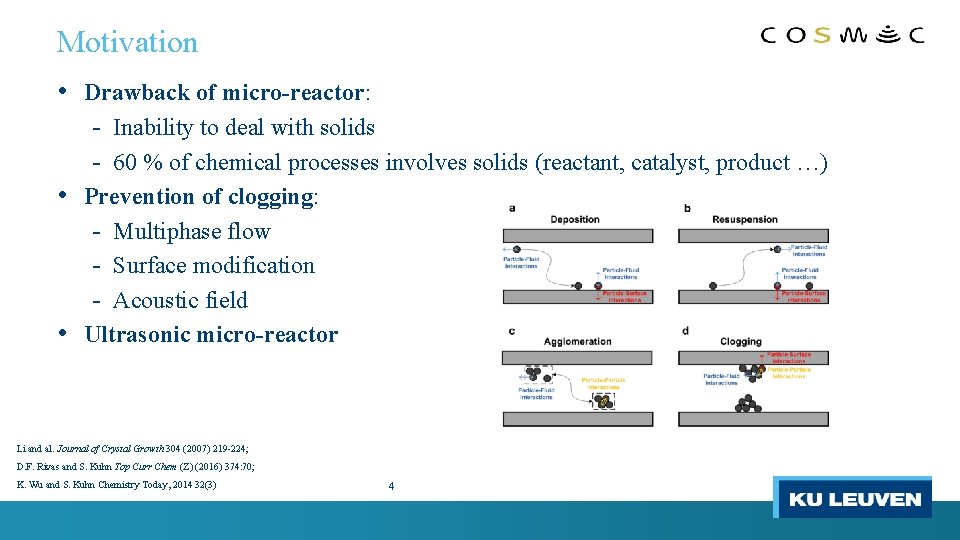
Motivation • Drawback of micro-reactor: - Inability to deal with solids - 60 % of chemical processes involves solids (reactant, catalyst, product …) • Prevention of clogging: - Multiphase flow - Surface modification - Acoustic field • Ultrasonic micro-reactor Li and al. Journal of Crystal Growth 304 (2007) 219 -224; D. F. Rivas and S. Kuhn Top Curr Chem (Z) (2016) 374: 70; K. Wu and S. Kuhn Chemistry Today, 2014 32(3) 4

Motivation Ultrasonic micro-reactor • Ultrasonic frequency range: 20 k. Hz to several GHz • Langevin’s type transducer: • Drawback: temperature increase Dong et al. AIChe, 2016, 62 (4), 1294 -1307; Chem. Commun. 2012, 48, 10935 -10947; Jordens et al. 5 Cryst. Growth Des. 2016, 6167 -6177

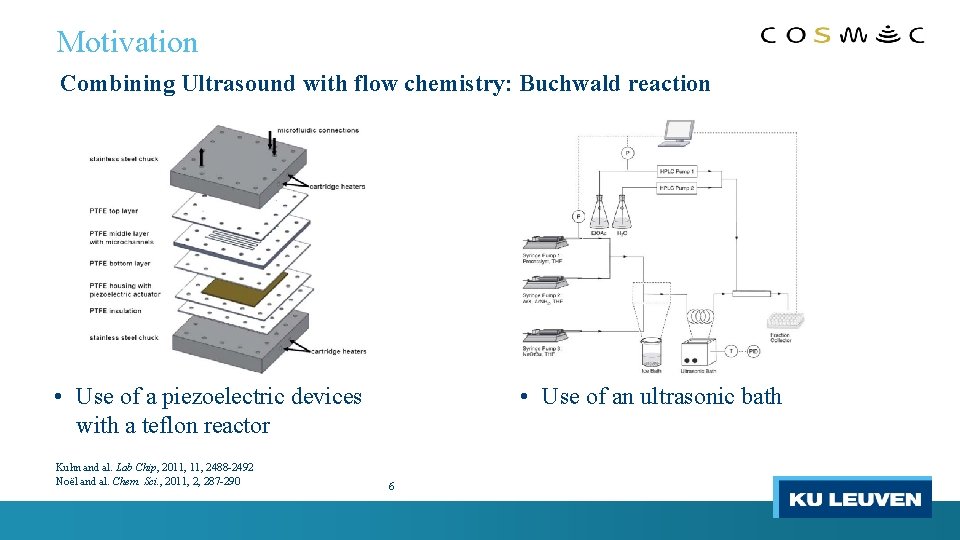
Motivation Combining Ultrasound with flow chemistry: Buchwald reaction • Use of a piezoelectric devices with a teflon reactor Kuhn and al. Lab Chip, 2011, 2488 -2492 Noël and al. Chem. Sci. , 2011, 2, 287 -290 • Use of an ultrasonic bath 6

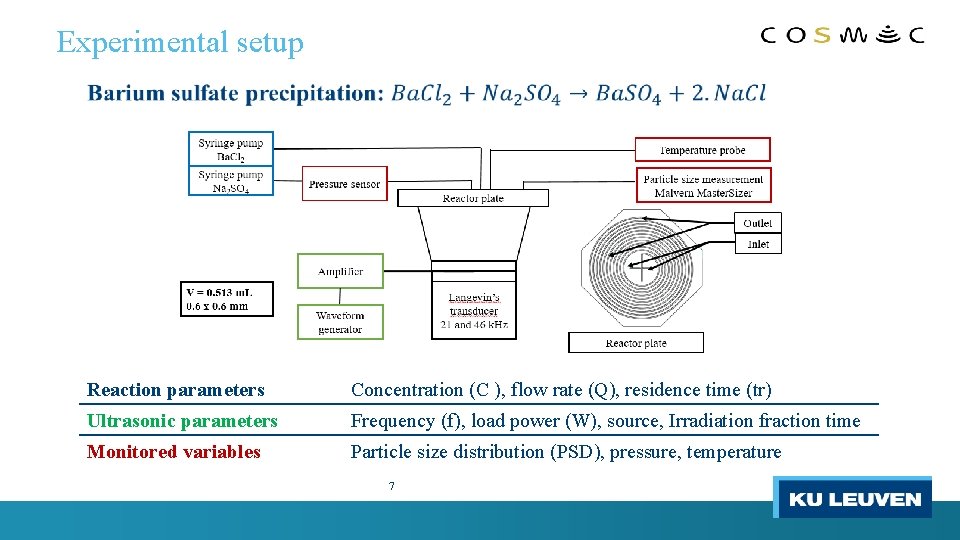
Experimental setup Reaction parameters Concentration (C ), flow rate (Q), residence time (tr) Ultrasonic parameters Frequency (f), load power (W), source, Irradiation fraction time Monitored variables Particle size distribution (PSD), pressure, temperature 7

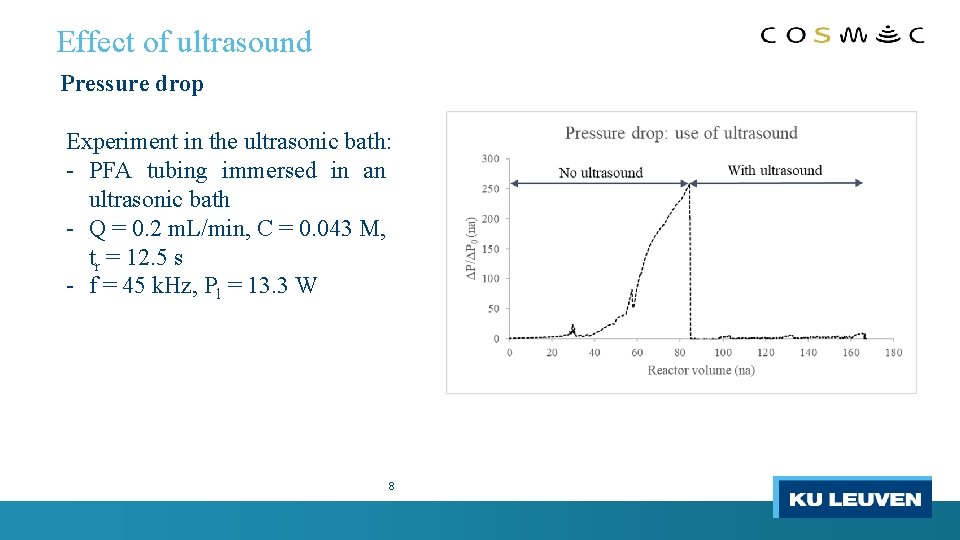
Effect of ultrasound Pressure drop Experiment in the ultrasonic bath: - PFA tubing immersed in an ultrasonic bath - Q = 0. 2 m. L/min, C = 0. 043 M, tr = 12. 5 s - f = 45 k. Hz, Pl = 13. 3 W 8

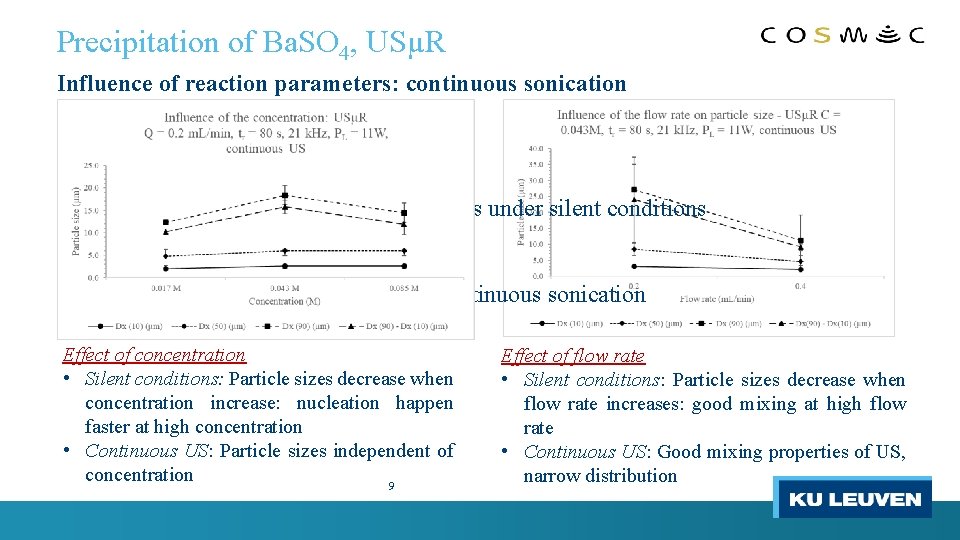
Precipitation of Ba. SO 4, USµR Influence of reaction parameters: continuous sonication Ø Preliminary study of reaction parameters under silent conditions Ø Effect of reaction parameters under continuous sonication Effect of concentration • Silent conditions: Particle sizes decrease when concentration increase: nucleation happen faster at high concentration • Continuous US: Particle sizes independent of concentration 9 Effect of flow rate • Silent conditions: Particle sizes decrease when flow rate increases: good mixing at high flow rate • Continuous US: Good mixing properties of US, narrow distribution

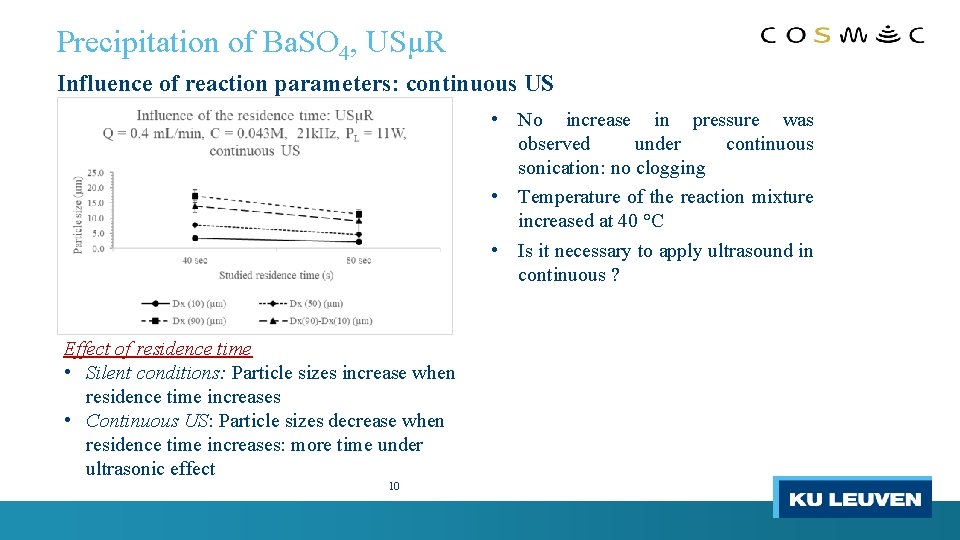
Precipitation of Ba. SO 4, USµR Influence of reaction parameters: continuous US • No increase in pressure was observed under continuous sonication: no clogging • Temperature of the reaction mixture increased at 40 °C • Is it necessary to apply ultrasound in continuous ? Effect of residence time • Silent conditions: Particle sizes increase when residence time increases • Continuous US: Particle sizes decrease when residence time increases: more time under ultrasonic effect 10

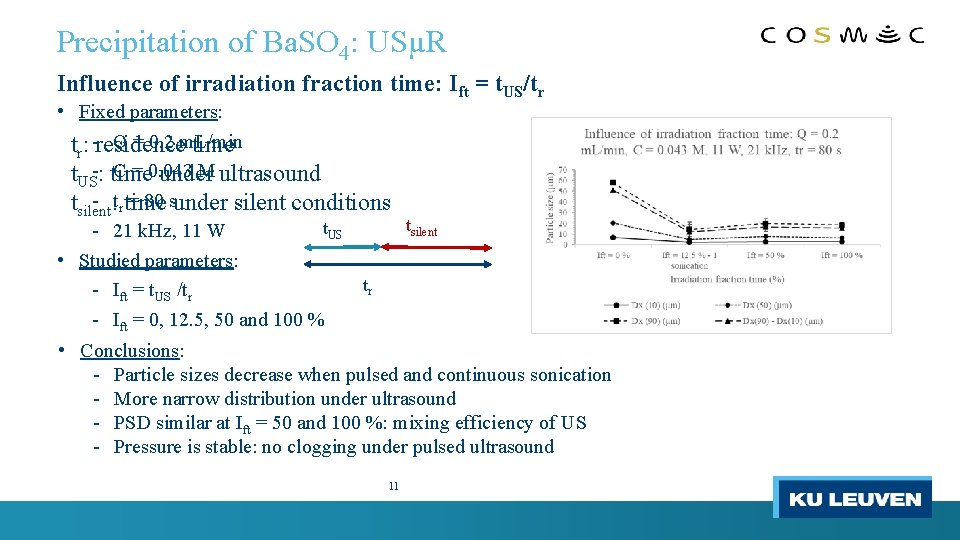
Precipitation of Ba. SO 4: USµR Influence of irradiation fraction time: Ift = t. US/tr • Fixed parameters: Q = 0. 2 m. L/min tr: -residence time C = 0. 043 M ultrasound t. US-: time under - : tr time = 80 sunder silent conditions tsilent t. US - 21 k. Hz, 11 W • Studied parameters: tr - Ift = t. US /tr - Ift = 0, 12. 5, 50 and 100 % tsilent • Conclusions: - Particle sizes decrease when pulsed and continuous sonication - More narrow distribution under ultrasound - PSD similar at Ift = 50 and 100 %: mixing efficiency of US - Pressure is stable: no clogging under pulsed ultrasound 11

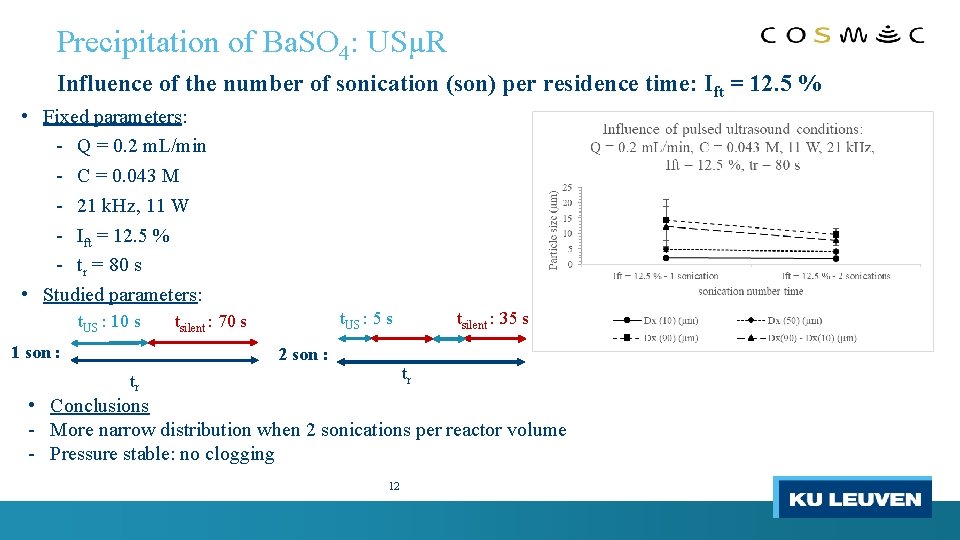
Precipitation of Ba. SO 4: USµR Influence of the number of sonication (son) per residence time: Ift = 12. 5 % • Fixed parameters: - Q = 0. 2 m. L/min - C = 0. 043 M - 21 k. Hz, 11 W - Ift = 12. 5 % - tr = 80 s • Studied parameters: t. US : 10 s 1 son : t. US : 5 s tsilent : 70 s 2 son : tsilent : 35 s tr tr • Conclusions - More narrow distribution when 2 sonications per reactor volume - Pressure stable: no clogging 12

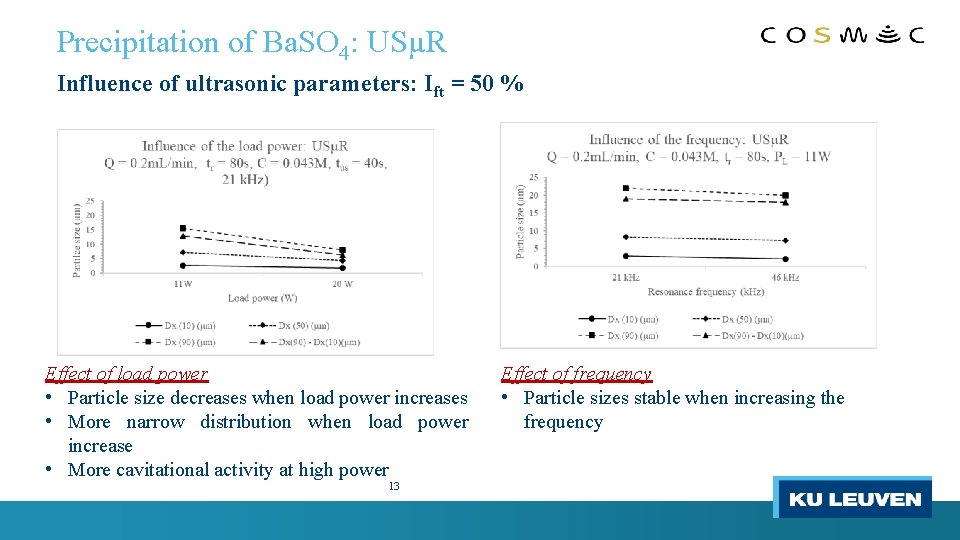
Precipitation of Ba. SO 4: USµR Influence of ultrasonic parameters: Ift = 50 % Effect of load power • Particle size decreases when load power increases • More narrow distribution when load power increase • More cavitational activity at high power 13 Effect of frequency • Particle sizes stable when increasing the frequency

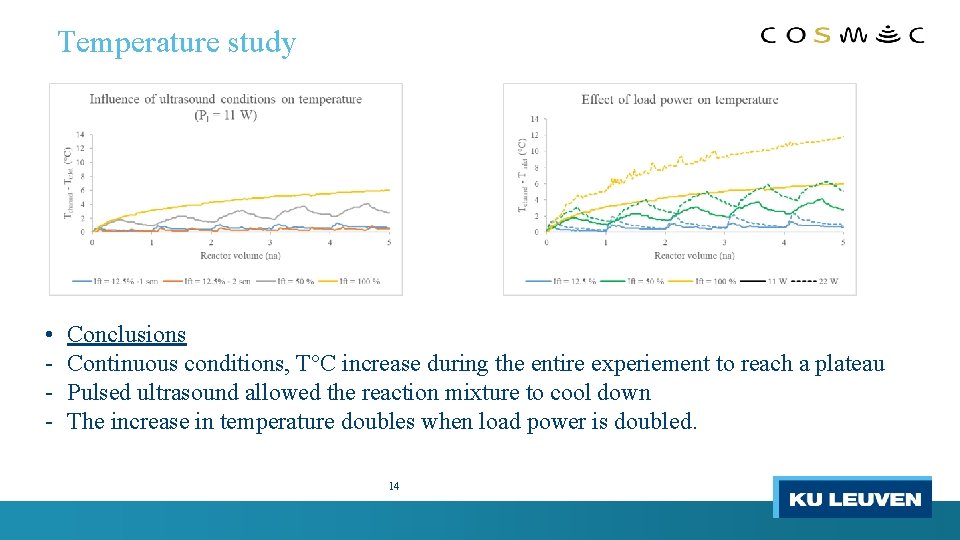
Temperature study • - Conclusions Continuous conditions, T°C increase during the entire experiement to reach a plateau Pulsed ultrasound allowed the reaction mixture to cool down The increase in temperature doubles when load power is doubled. 14

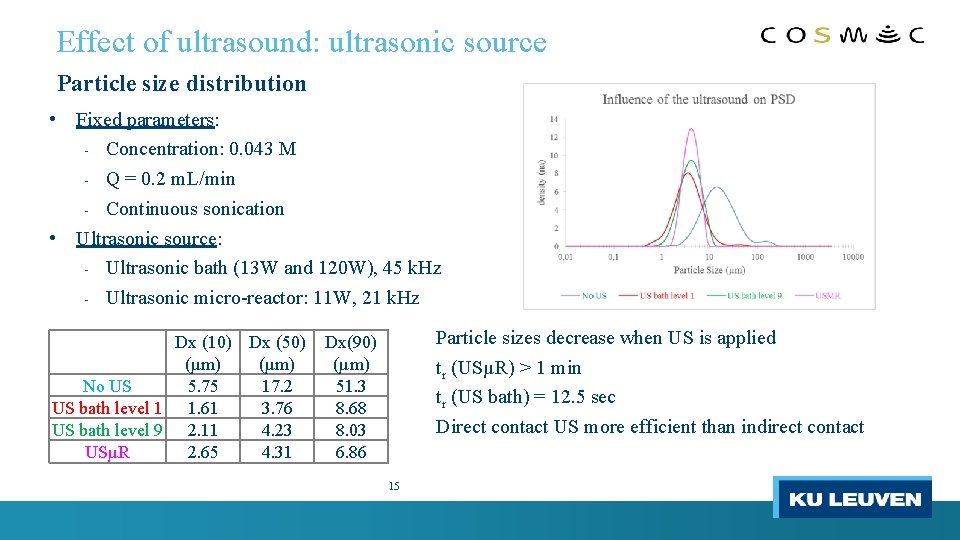
Effect of ultrasound: ultrasonic source Particle size distribution • Fixed parameters: Concentration: 0. 043 M - Q = 0. 2 m. L/min - Continuous sonication • Ultrasonic source: - Ultrasonic bath (13 W and 120 W), 45 k. Hz - Ultrasonic micro-reactor: 11 W, 21 k. Hz - Dx (10) Dx (50) (µm) No US 5. 75 17. 2 US bath level 1 1. 61 3. 76 US bath level 9 2. 11 4. 23 USµR 2. 65 4. 31 Particle sizes decrease when US is applied tr (USµR) > 1 min tr (US bath) = 12. 5 sec Direct contact US more efficient than indirect contact Dx(90) (µm) 51. 3 8. 68 8. 03 6. 86 15

Conclusion • Applying continuous or pulsed ultrasound allowed to prevent clogging in micro-channel • Changing ultrasonic parameters allowed to deliver particles with different size distributions • Ultrasound enhances mixing properties • Pulsed ultrasound allowed to have a certain control of the reaction mixture temperature 16

Acknowledgment This project has received fundig from the European Union’s EU Framework Programme for Research and Innovation Horizon 2020 under the Grant Agreement No 721290 https: //cosmic-etn. eu/ 17

Thank you for your attention 18

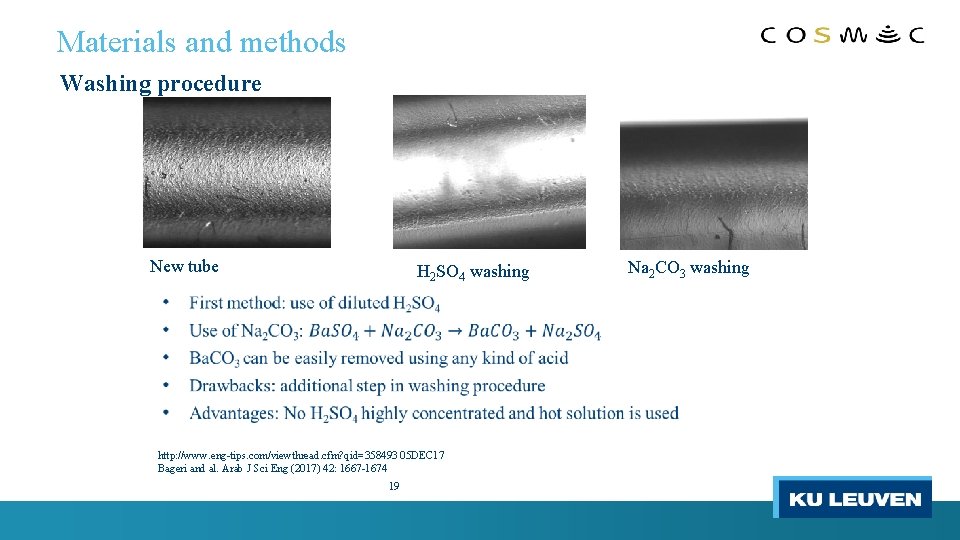
Materials and methods Washing procedure New tube H 2 SO 4 washing http: //www. eng-tips. com/viewthread. cfm? qid=358493 05 DEC 17 Bageri and al. Arab J Sci Eng (2017) 42: 1667 -1674 19 Na 2 CO 3 washing