A LEVEL ELECTRON ARRANGEMENTS OBJECTIVES To describe how

A LEVEL ELECTRON ARRANGEMENTS OBJECTIVES: To describe how electrons fill energy levels, including the s, p, d and f sub-levels To explain how chromium and copper are ‘different’ SUMMARY SO FAR… KEY WORDS: Write a 3 sentence summary of electrons as you know them at present ENERGY LEVEL ORBITAL SUB-LEVEL SPIN
A LEVEL ELECTRON ARRANGEMENTS Shells • Each shell you know from GCSE is split into sub-shells, each with slightly different energies • These sub-shells are called s, p, d and f sub-shells • Each subshell has orbitals. • Each orbital can hold 2 electrons • Copy the table from page 18 and describe in one sentence what it shows you

A LEVEL ELECTRON ARRANGEMENTS Sub-Shell Notation • There is a way of writing electron configuration you HAVE TO KNOW!!! • Neon has 10 electrons, so write it using sub-shell notation: • Now try this with Lithium, then Nitrogen and oxygen • And finally, Calcium…
A LEVEL ELECTRON ARRANGEMENTS ENERGY LEVELS • Electrons have fixed energies, they move around the nucleus in regions called shells or energy levels • Different shells have differing amounts of energy. Level 1 contains electrons closest to the nucleus. These have the lowest energy • Not all the electrons in a shell have exactly the same amount of energy. • Shells are divided into sub-shells that have slightly different energies • Sub shells have orbitals which can each hold up to 2 electrons
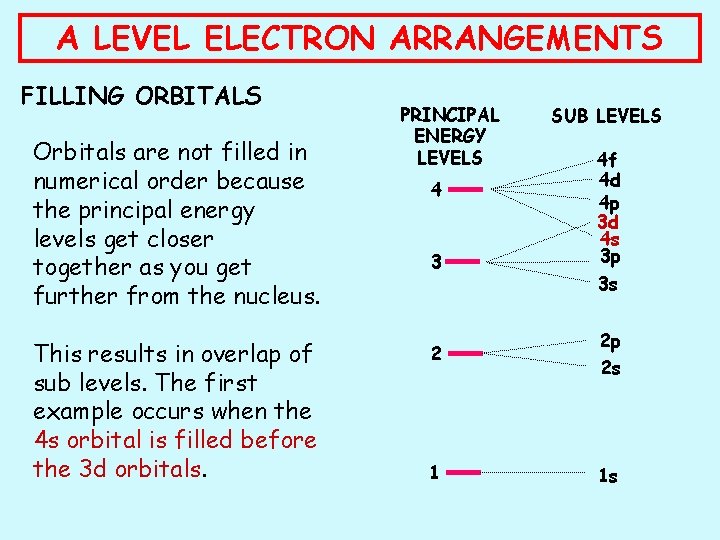
A LEVEL ELECTRON ARRANGEMENTS FILLING ORBITALS Orbitals are not filled in numerical order because the principal energy levels get closer together as you get further from the nucleus. This results in overlap of sub levels. The first example occurs when the 4 s orbital is filled before the 3 d orbitals. PRINCIPAL ENERGY LEVELS 4 3 SUB LEVELS 4 f 4 d 4 p 3 d 4 s 3 p 3 s 2 2 p 2 s 1 1 s

A LEVEL ELECTRON ARRANGEMENTS HOW TO REMEMBER THE FILLING ORDER 1 s RULES FOR FILLING ORBITALS: 1. Fill up lowest energy sub levels first (Aufbau Principle) 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f 5 s 5 p 5 d 5 f 6 s 6 p 6 d 7 s 7 p 2. Fill orbitals singly at first before doubling (Hund’s Rule) 3. Remember that 4 s is filled before 3 d!
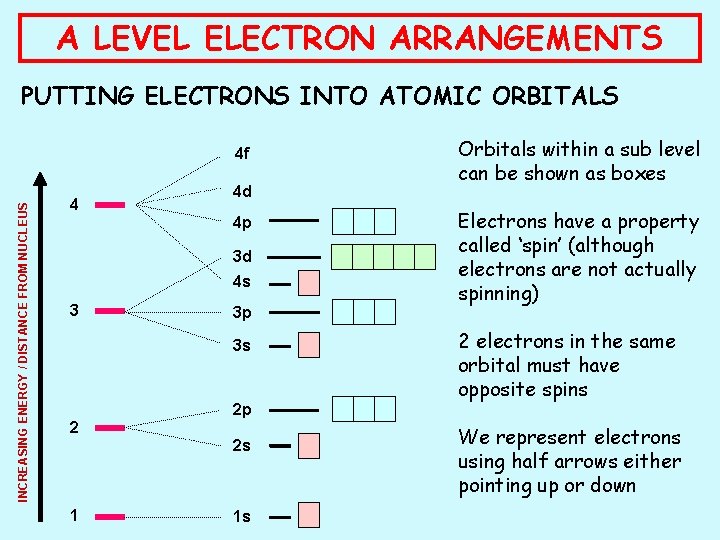
A LEVEL ELECTRON ARRANGEMENTS PUTTING ELECTRONS INTO ATOMIC ORBITALS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s 2 2 p 2 s 1 1 s Orbitals within a sub level can be shown as boxes Electrons have a property called ‘spin’ (although electrons are not actually spinning) 2 electrons in the same orbital must have opposite spins We represent electrons using half arrows either pointing up or down
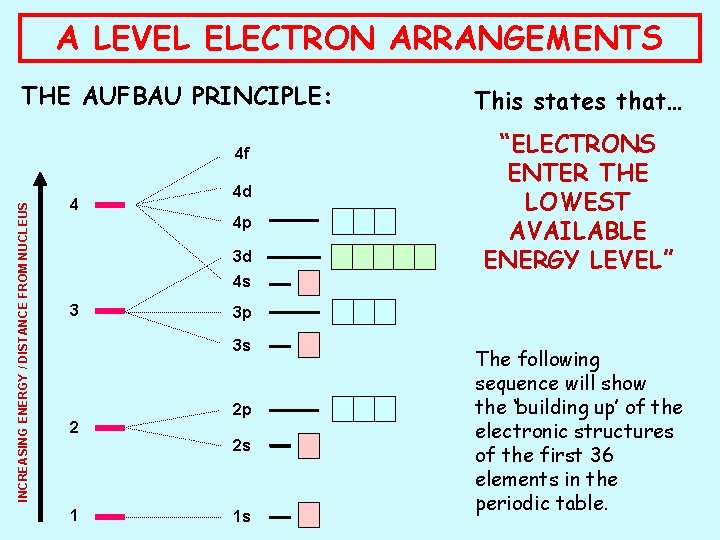
A LEVEL ELECTRON ARRANGEMENTS THE AUFBAU PRINCIPLE: INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 2 p 2 s 1 “ELECTRONS ENTER THE LOWEST AVAILABLE ENERGY LEVEL” 3 p 3 s 2 This states that… 1 s The following sequence will show the ‘building up’ of the electronic structures of the first 36 elements in the periodic table.
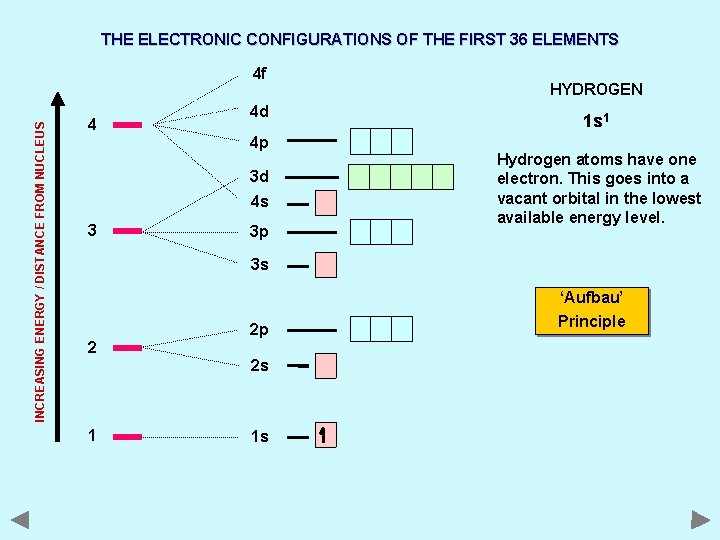
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p HYDROGEN 1 s 1 Hydrogen atoms have one electron. This goes into a vacant orbital in the lowest available energy level. 3 s ‘Aufbau’ 2 2 p 2 s 1 1 s Principle
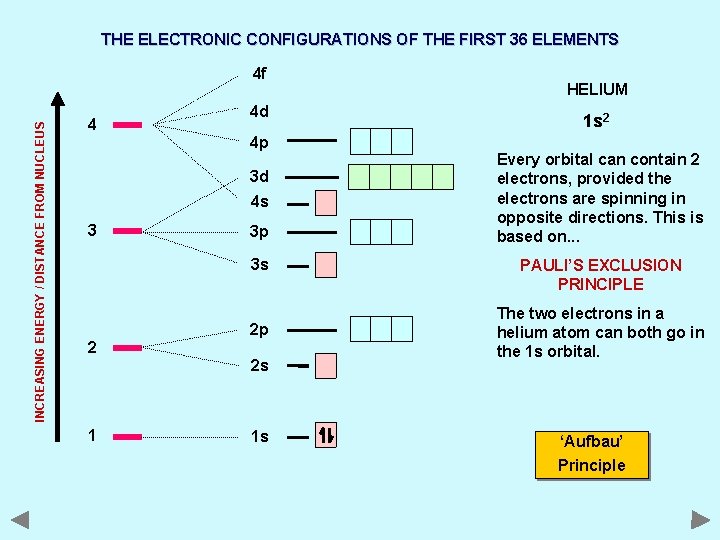
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s 2 2 p 2 s 1 1 s HELIUM 1 s 2 Every orbital can contain 2 electrons, provided the electrons are spinning in opposite directions. This is based on. . . PAULI’S EXCLUSION PRINCIPLE The two electrons in a helium atom can both go in the 1 s orbital. ‘Aufbau’ Principle
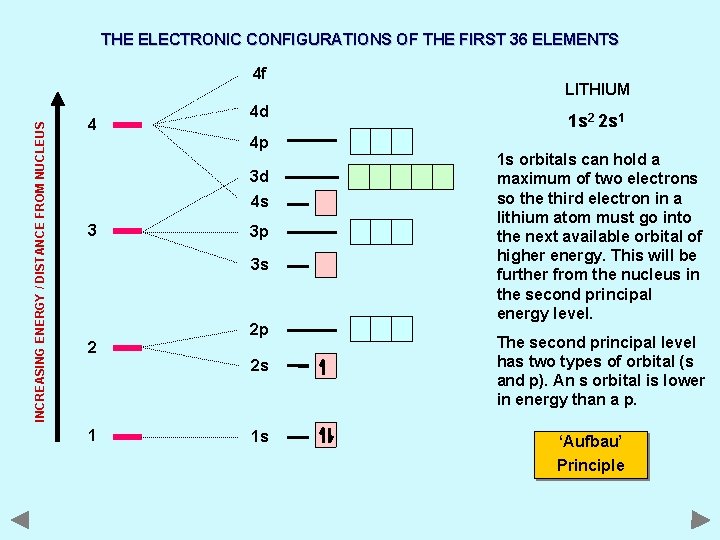
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s 2 2 p 2 s 1 1 s LITHIUM 1 s 2 2 s 1 1 s orbitals can hold a maximum of two electrons so the third electron in a lithium atom must go into the next available orbital of higher energy. This will be further from the nucleus in the second principal energy level. The second principal level has two types of orbital (s and p). An s orbital is lower in energy than a p. ‘Aufbau’ Principle
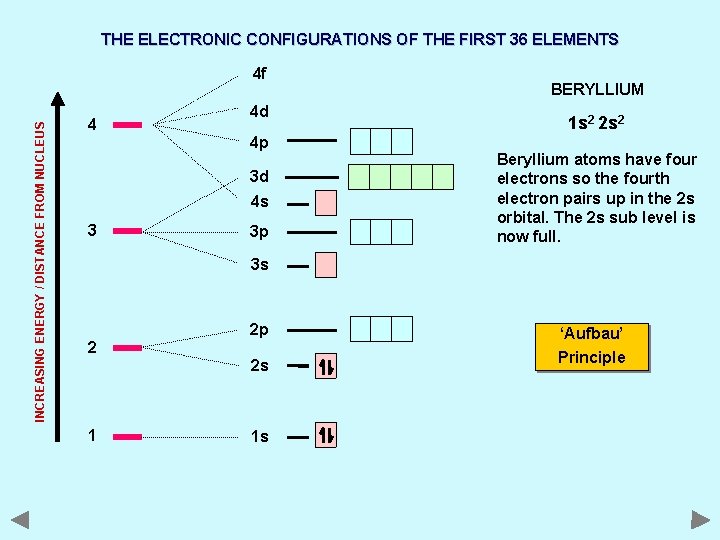
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p BERYLLIUM 1 s 2 2 s 2 Beryllium atoms have four electrons so the fourth electron pairs up in the 2 s orbital. The 2 s sub level is now full. 3 s 2 2 p 2 s 1 1 s ‘Aufbau’ Principle
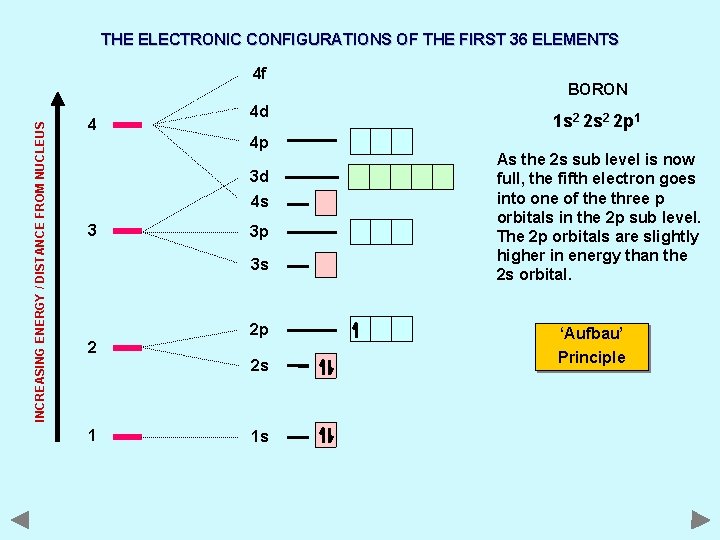
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s 2 2 p 2 s 1 1 s BORON 1 s 2 2 p 1 As the 2 s sub level is now full, the fifth electron goes into one of the three p orbitals in the 2 p sub level. The 2 p orbitals are slightly higher in energy than the 2 s orbital. ‘Aufbau’ Principle
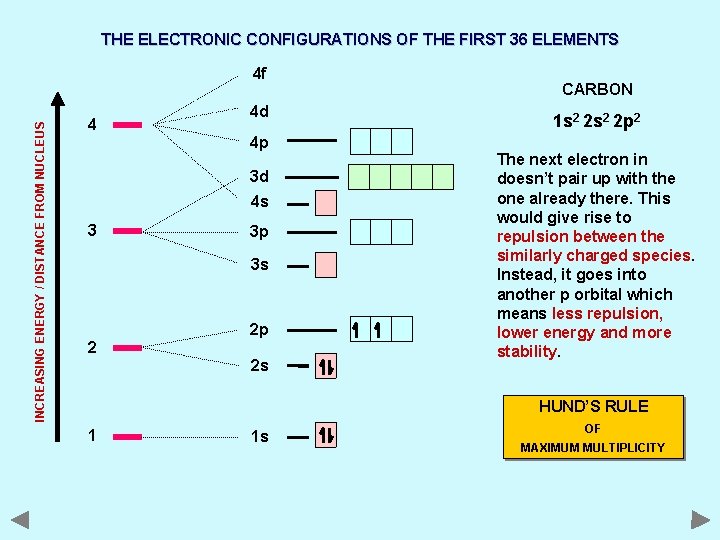
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s 2 2 p 2 s CARBON 1 s 2 2 p 2 The next electron in doesn’t pair up with the one already there. This would give rise to repulsion between the similarly charged species. Instead, it goes into another p orbital which means less repulsion, lower energy and more stability. HUND’S RULE 1 1 s OF MAXIMUM MULTIPLICITY
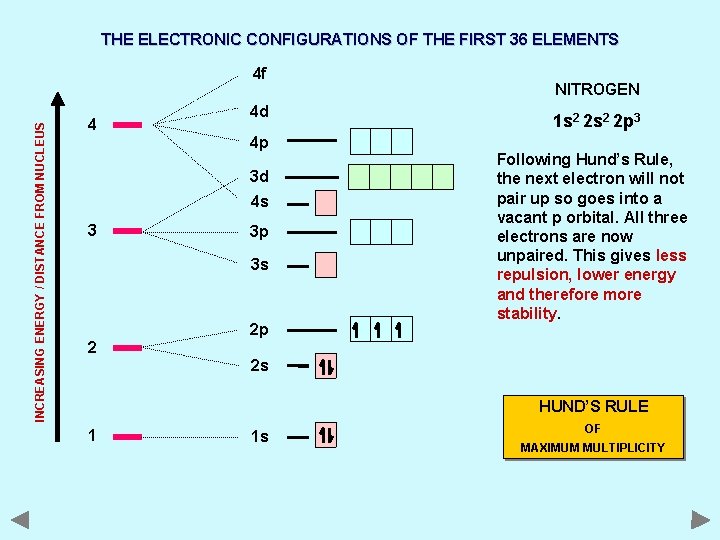
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s 2 2 p NITROGEN 1 s 2 2 p 3 Following Hund’s Rule, the next electron will not pair up so goes into a vacant p orbital. All three electrons are now unpaired. This gives less repulsion, lower energy and therefore more stability. 2 s HUND’S RULE 1 1 s OF MAXIMUM MULTIPLICITY
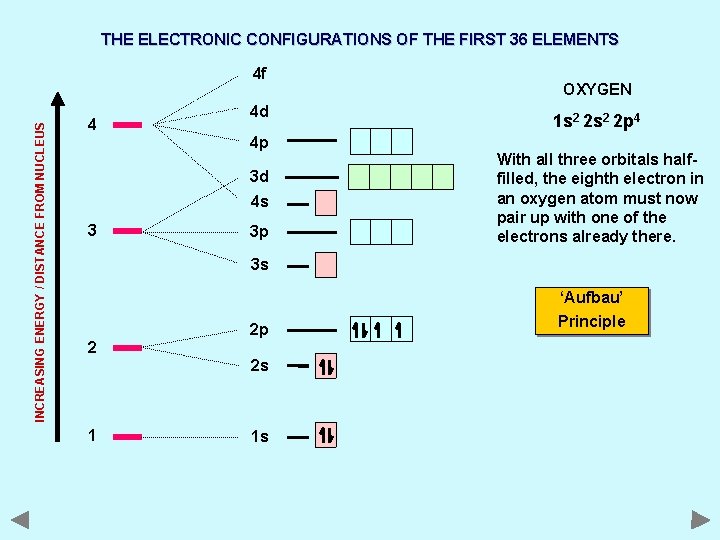
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p OXYGEN 1 s 2 2 p 4 With all three orbitals halffilled, the eighth electron in an oxygen atom must now pair up with one of the electrons already there. 3 s ‘Aufbau’ 2 2 p 2 s 1 1 s Principle
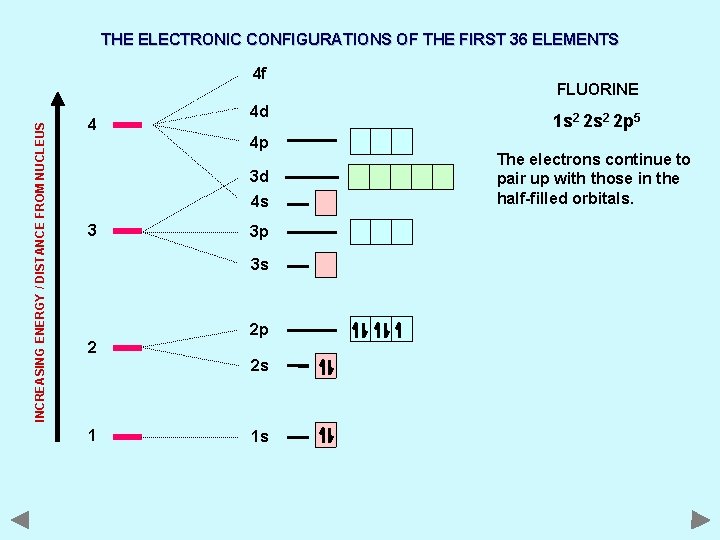
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s 2 2 p 2 s 1 1 s FLUORINE 1 s 2 2 p 5 The electrons continue to pair up with those in the half-filled orbitals.
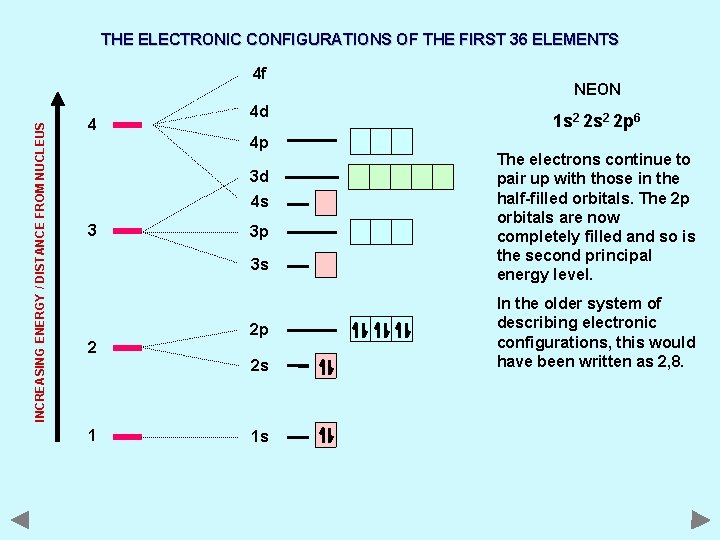
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s 2 2 p 2 s 1 1 s NEON 1 s 2 2 p 6 The electrons continue to pair up with those in the half-filled orbitals. The 2 p orbitals are now completely filled and so is the second principal energy level. In the older system of describing electronic configurations, this would have been written as 2, 8.
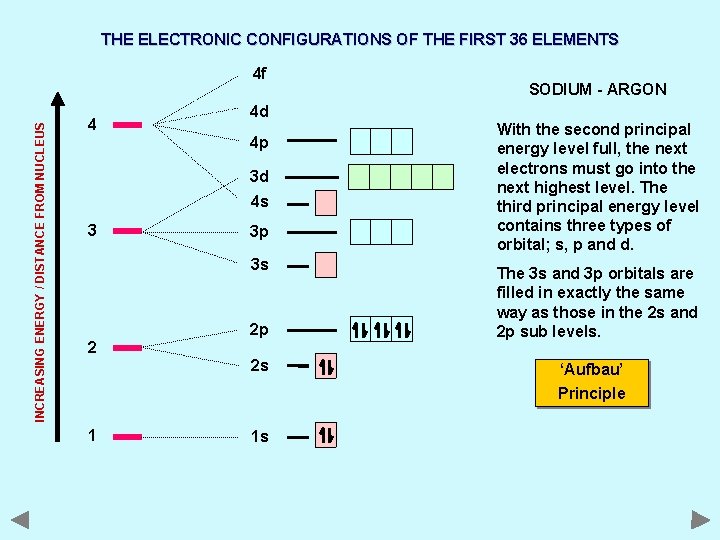
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s 2 SODIUM - ARGON 2 p 2 s With the second principal energy level full, the next electrons must go into the next highest level. The third principal energy level contains three types of orbital; s, p and d. The 3 s and 3 p orbitals are filled in exactly the same way as those in the 2 s and 2 p sub levels. ‘Aufbau’ Principle 1 1 s
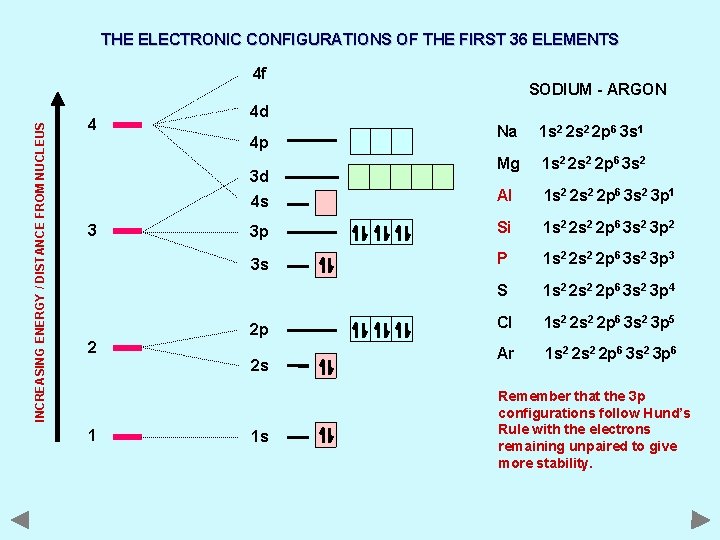
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d Na 1 s 2 2 p 6 3 s 1 Mg 1 s 2 2 p 6 3 s 2 4 s Al 1 s 2 2 p 6 3 s 2 3 p 1 3 p Si 1 s 2 2 p 6 3 s 2 3 p 2 3 s P 1 s 2 2 p 6 3 s 2 3 p 3 S 1 s 2 2 p 6 3 s 2 3 p 4 Cl 1 s 2 2 p 6 3 s 2 3 p 5 Ar 1 s 2 2 p 6 3 s 2 3 p 6 4 p 3 d 3 2 2 p 2 s 1 SODIUM - ARGON 1 s Remember that the 3 p configurations follow Hund’s Rule with the electrons remaining unpaired to give more stability.
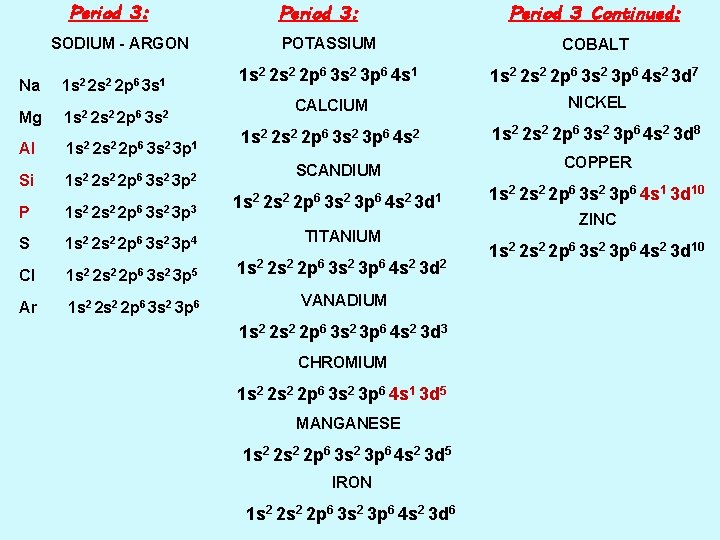
Period 3: SODIUM - ARGON Na 1 s 2 2 p 6 3 s 1 Mg 1 s 2 2 p 6 3 s 2 Al Si 1 s 2 2 p 6 3 s 2 3 p 1 1 s 2 2 p 6 3 s 2 3 p 2 Period 3: Period 3 Continued: POTASSIUM COBALT 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 7 CALCIUM NICKEL 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 8 SCANDIUM COPPER 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 1 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10 P 1 s 2 2 p 6 3 s 2 3 p 3 S 1 s 2 2 p 6 3 s 2 3 p 4 TITANIUM Cl 1 s 2 2 p 6 3 s 2 3 p 5 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 2 Ar 1 s 2 2 p 6 3 s 2 3 p 6 VANADIUM 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 3 CHROMIUM 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5 MANGANESE 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 5 IRON 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 ZINC 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10

Transition Metals WALT: Be able to draw arrow boxes on energy level diagrams for Transition metals Know the two ‘Special’ cases
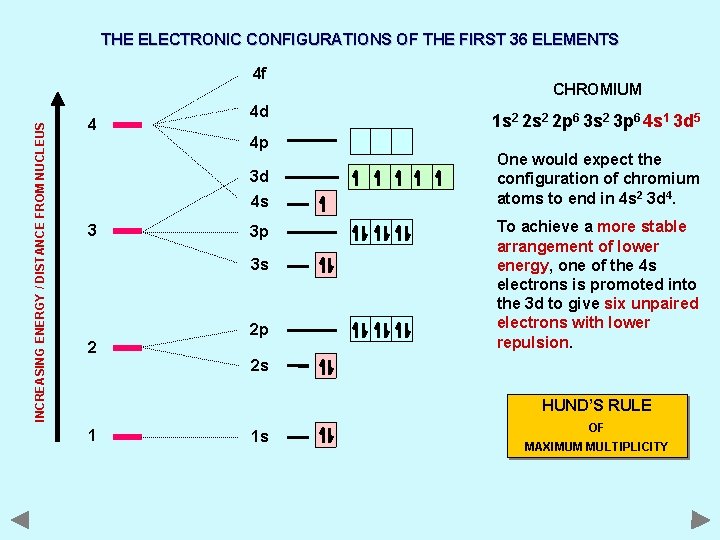
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s 2 2 p CHROMIUM 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5 One would expect the configuration of chromium atoms to end in 4 s 2 3 d 4. To achieve a more stable arrangement of lower energy, one of the 4 s electrons is promoted into the 3 d to give six unpaired electrons with lower repulsion. 2 s HUND’S RULE 1 1 s OF MAXIMUM MULTIPLICITY
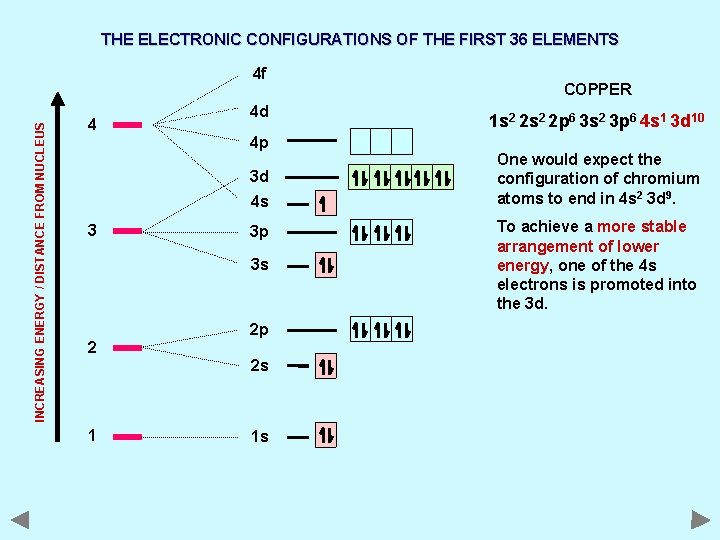
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s 2 2 p 2 s 1 1 s COPPER 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10 One would expect the configuration of chromium atoms to end in 4 s 2 3 d 9. To achieve a more stable arrangement of lower energy, one of the 4 s electrons is promoted into the 3 d.

Period 4: Period 4 Continued: SCANDIUM COBALT 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 1 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 7 TITANIUM 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 2 NICKEL 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 8 VANADIUM 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 3 ZINC 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 MANGANESE 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 5 IRON 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6

Period 4: Period 4 Continued: POTASSIUM COBALT 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 7 CALCIUM NICKEL 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 8 SCANDIUM COPPER 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 1 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10 TITANIUM 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 2 VANADIUM 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 3 CHROMIUM 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5 MANGANESE 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 5 IRON 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 ZINC 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10
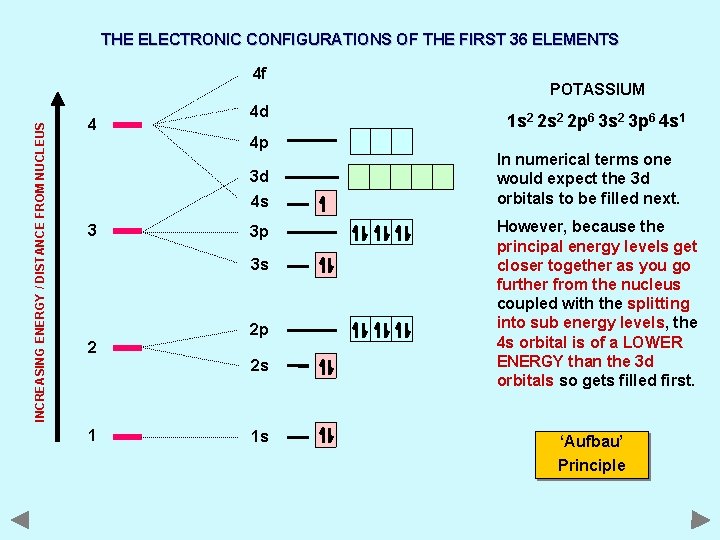
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s 2 2 p 2 s 1 1 s POTASSIUM 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 In numerical terms one would expect the 3 d orbitals to be filled next. However, because the principal energy levels get closer together as you go further from the nucleus coupled with the splitting into sub energy levels, the 4 s orbital is of a LOWER ENERGY than the 3 d orbitals so gets filled first. ‘Aufbau’ Principle
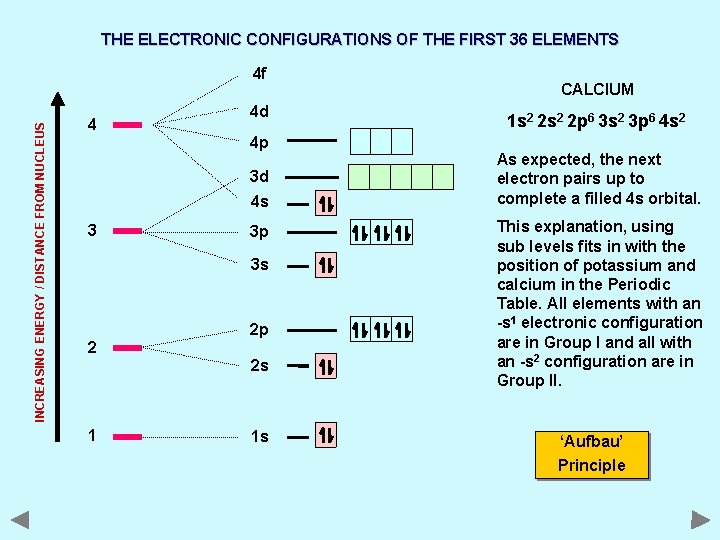
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s 2 2 p 2 s 1 1 s CALCIUM 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 As expected, the next electron pairs up to complete a filled 4 s orbital. This explanation, using sub levels fits in with the position of potassium and calcium in the Periodic Table. All elements with an -s 1 electronic configuration are in Group I and all with an -s 2 configuration are in Group II. ‘Aufbau’ Principle
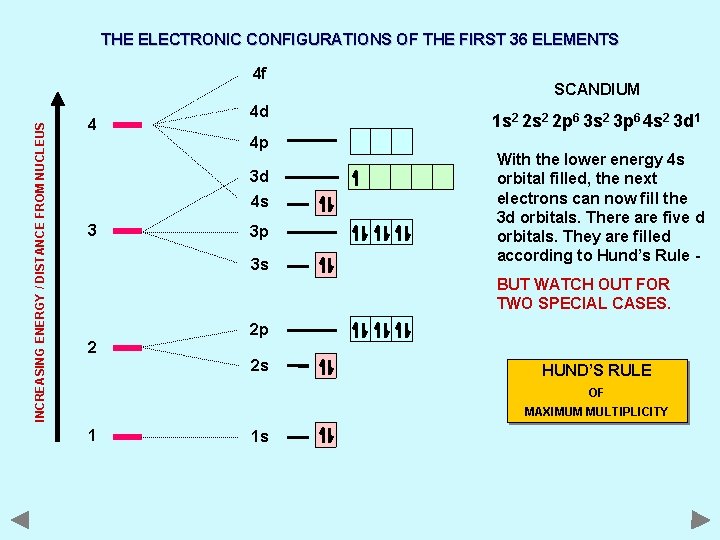
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s SCANDIUM 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 1 With the lower energy 4 s orbital filled, the next electrons can now fill the 3 d orbitals. There are five d orbitals. They are filled according to Hund’s Rule BUT WATCH OUT FOR TWO SPECIAL CASES. 2 2 p 2 s HUND’S RULE OF MAXIMUM MULTIPLICITY 1 1 s
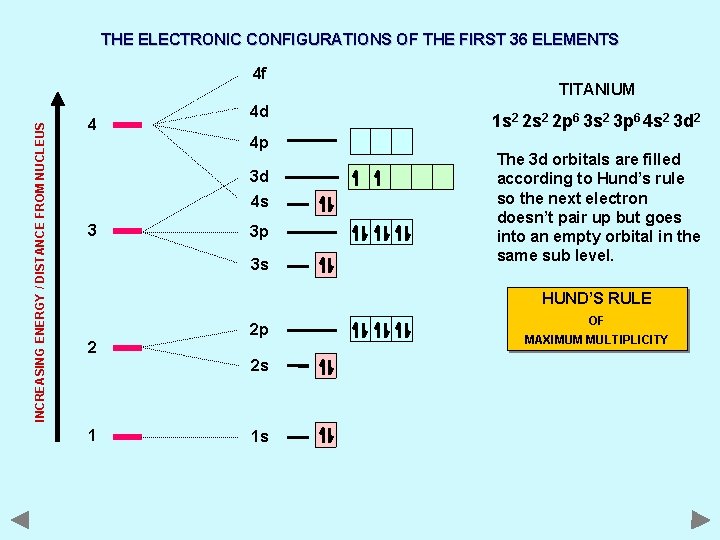
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s TITANIUM 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 2 The 3 d orbitals are filled according to Hund’s rule so the next electron doesn’t pair up but goes into an empty orbital in the same sub level. HUND’S RULE 2 2 p 2 s 1 1 s OF MAXIMUM MULTIPLICITY
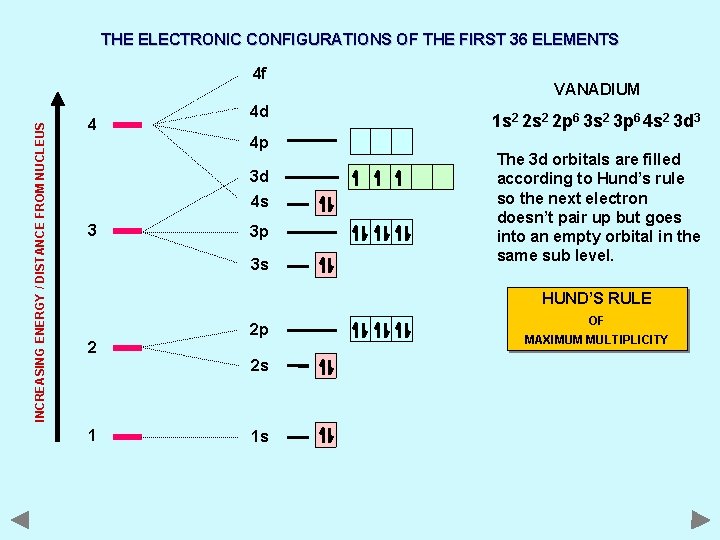
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s VANADIUM 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 3 The 3 d orbitals are filled according to Hund’s rule so the next electron doesn’t pair up but goes into an empty orbital in the same sub level. HUND’S RULE 2 2 p 2 s 1 1 s OF MAXIMUM MULTIPLICITY
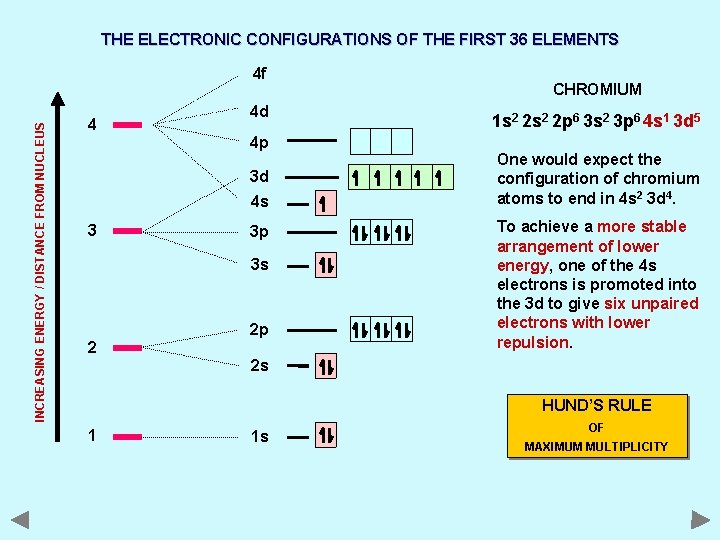
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s 2 2 p CHROMIUM 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5 One would expect the configuration of chromium atoms to end in 4 s 2 3 d 4. To achieve a more stable arrangement of lower energy, one of the 4 s electrons is promoted into the 3 d to give six unpaired electrons with lower repulsion. 2 s HUND’S RULE 1 1 s OF MAXIMUM MULTIPLICITY
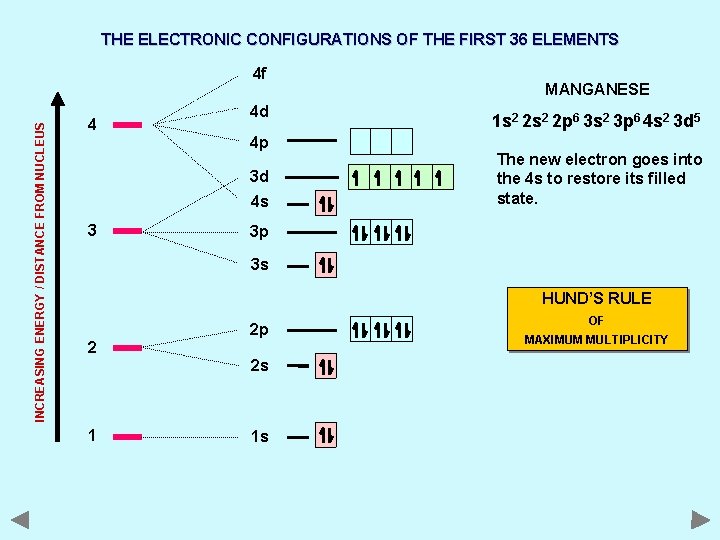
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 MANGANESE 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 5 The new electron goes into the 4 s to restore its filled state. 3 p 3 s HUND’S RULE 2 2 p 2 s 1 1 s OF MAXIMUM MULTIPLICITY
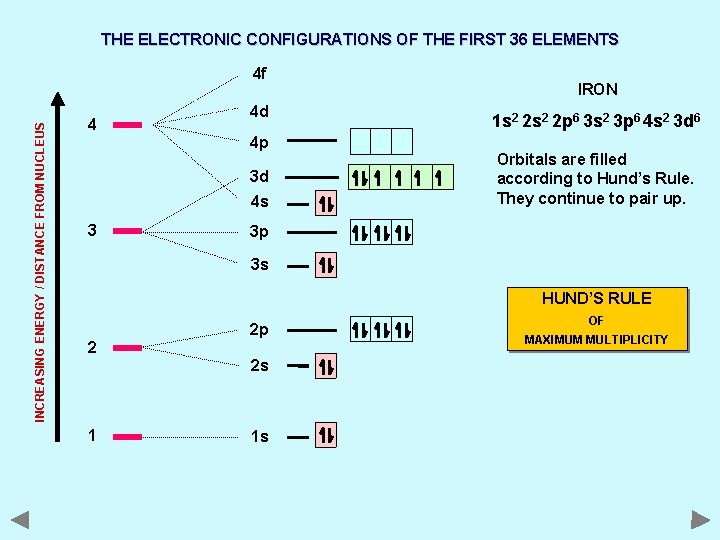
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 IRON 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 Orbitals are filled according to Hund’s Rule. They continue to pair up. 3 p 3 s HUND’S RULE 2 2 p 2 s 1 1 s OF MAXIMUM MULTIPLICITY
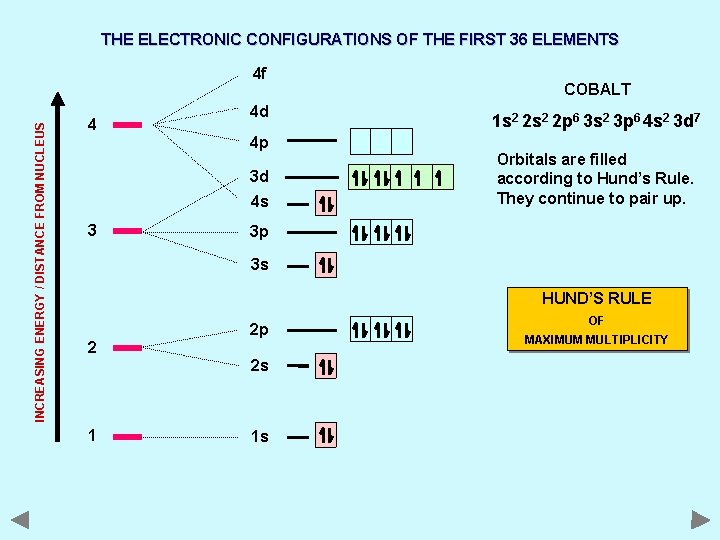
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 COBALT 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 7 Orbitals are filled according to Hund’s Rule. They continue to pair up. 3 p 3 s HUND’S RULE 2 2 p 2 s 1 1 s OF MAXIMUM MULTIPLICITY
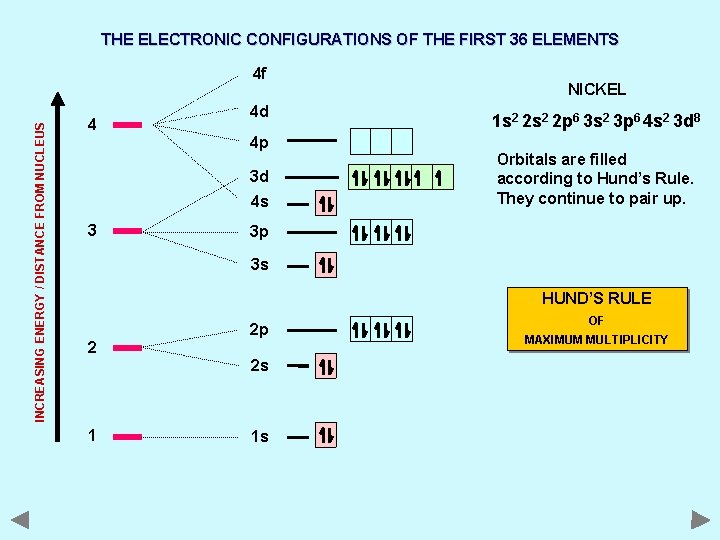
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 NICKEL 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 8 Orbitals are filled according to Hund’s Rule. They continue to pair up. 3 p 3 s HUND’S RULE 2 2 p 2 s 1 1 s OF MAXIMUM MULTIPLICITY
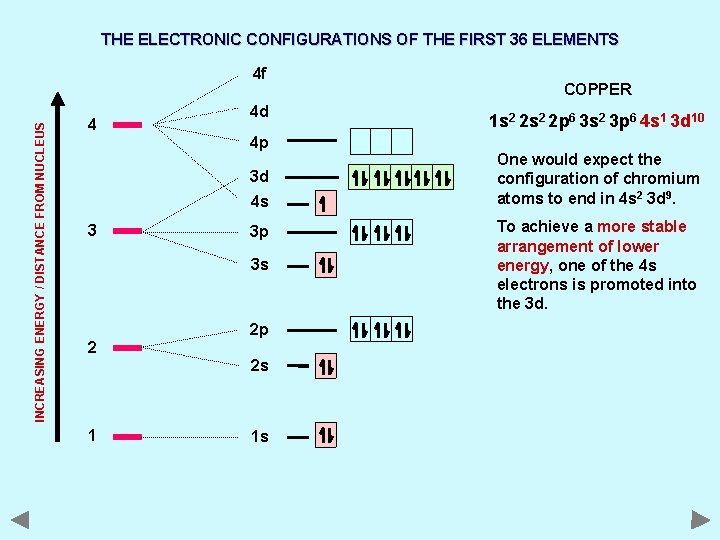
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s 2 2 p 2 s 1 1 s COPPER 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10 One would expect the configuration of chromium atoms to end in 4 s 2 3 d 9. To achieve a more stable arrangement of lower energy, one of the 4 s electrons is promoted into the 3 d.
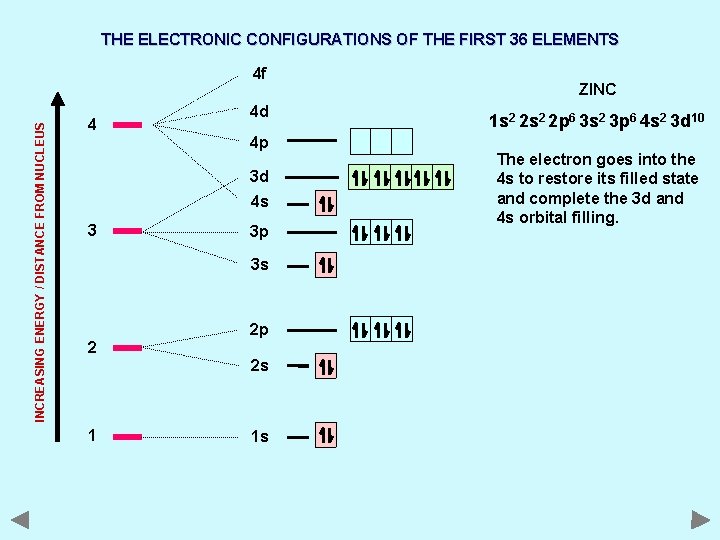
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s 2 2 p 2 s 1 1 s ZINC 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 The electron goes into the 4 s to restore its filled state and complete the 3 d and 4 s orbital filling.
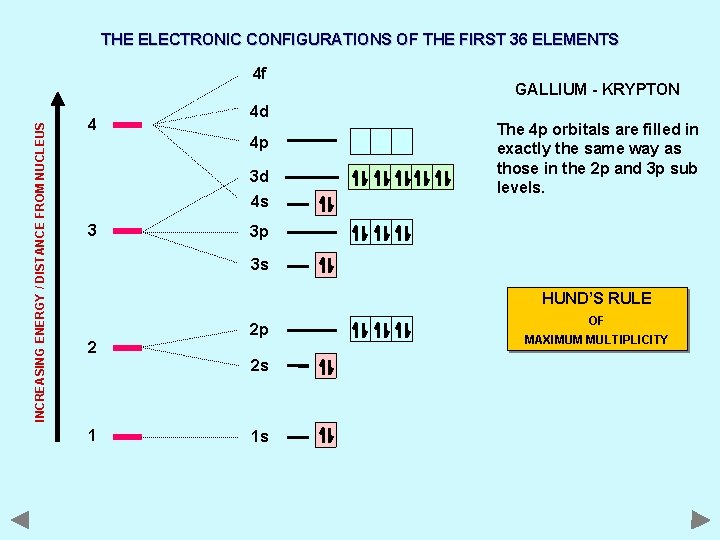
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 GALLIUM - KRYPTON The 4 p orbitals are filled in exactly the same way as those in the 2 p and 3 p sub levels. 3 p 3 s HUND’S RULE 2 2 p 2 s 1 1 s OF MAXIMUM MULTIPLICITY
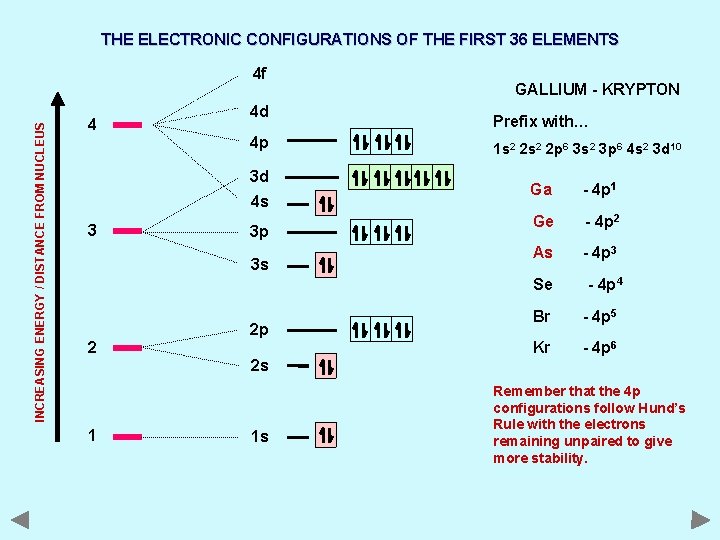
THE ELECTRONIC CONFIGURATIONS OF THE FIRST 36 ELEMENTS INCREASING ENERGY / DISTANCE FROM NUCLEUS 4 f 4 4 d 4 p 3 d 4 s 3 3 p 3 s GALLIUM - KRYPTON Prefix with… 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 Ga - 4 p 1 Ge - 4 p 2 As - 4 p 3 Se 2 2 p 2 s 1 1 s - 4 p 4 Br - 4 p 5 Kr - 4 p 6 Remember that the 4 p configurations follow Hund’s Rule with the electrons remaining unpaired to give more stability.
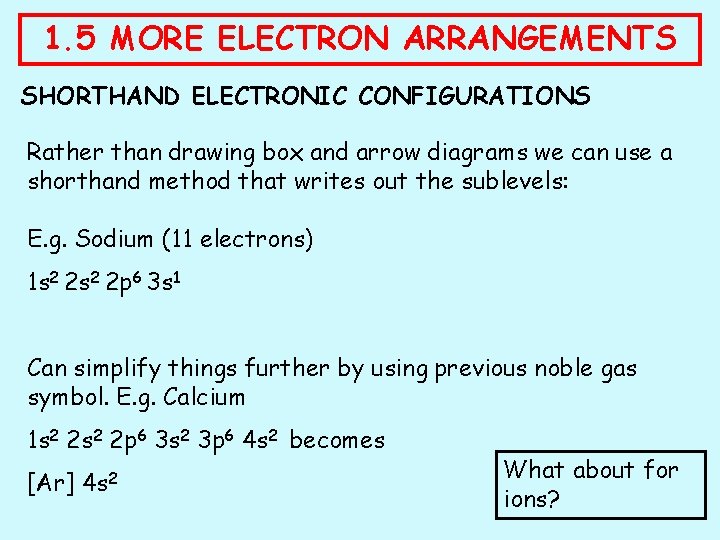
1. 5 MORE ELECTRON ARRANGEMENTS SHORTHAND ELECTRONIC CONFIGURATIONS Rather than drawing box and arrow diagrams we can use a shorthand method that writes out the sublevels: E. g. Sodium (11 electrons) 1 s 2 2 p 6 3 s 1 Can simplify things further by using previous noble gas symbol. E. g. Calcium 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 becomes [Ar] 4 s 2 What about for ions?

1. 5 MORE ELECTRON ARRANGEMENTS OVER TO YOU Draw spin diagrams for the first 36 elements [Remember be careful with chromium and copper!] Add in the full shorthand electronic configurations Come up to the front to mark your work when done EXTENSION: Summary Questions on page 16
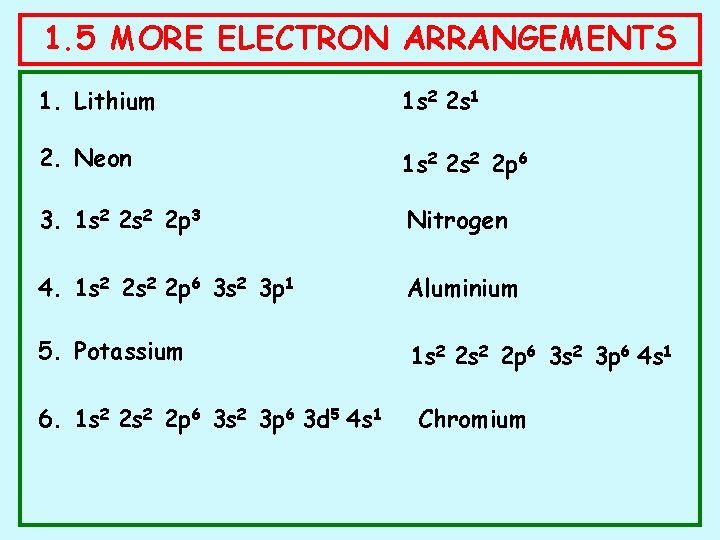
1. 5 MORE ELECTRON ARRANGEMENTS 1. Lithium 1 s 2 2 s 1 2. Neon 1 s 2 2 p 6 3. 1 s 2 2 p 3 Nitrogen 4. 1 s 2 2 p 6 3 s 2 3 p 1 Aluminium 5. Potassium 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 6. 1 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 Chromium

1. 5 MORE ELECTRON ARRANGEMENTS CHAMPIONS LEAGUE FINAL Top scorer from each group now go head to be crowned Electron King/Queen First correct whiteboard placed in front of me wins Q: Write the shorthand for Copper 1 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 1
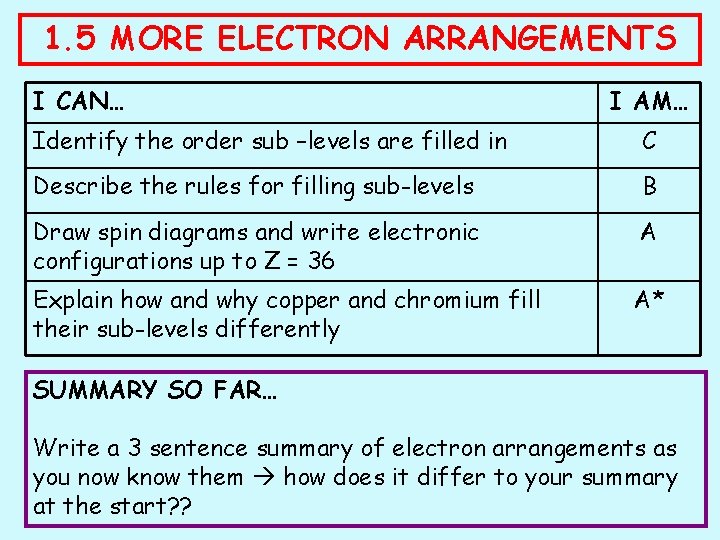
1. 5 MORE ELECTRON ARRANGEMENTS I CAN… I AM… Identify the order sub –levels are filled in C Describe the rules for filling sub-levels B Draw spin diagrams and write electronic configurations up to Z = 36 A Explain how and why copper and chromium fill their sub-levels differently A* SUMMARY SO FAR… Write a 3 sentence summary of electron arrangements as you now know them how does it differ to your summary at the start? ?
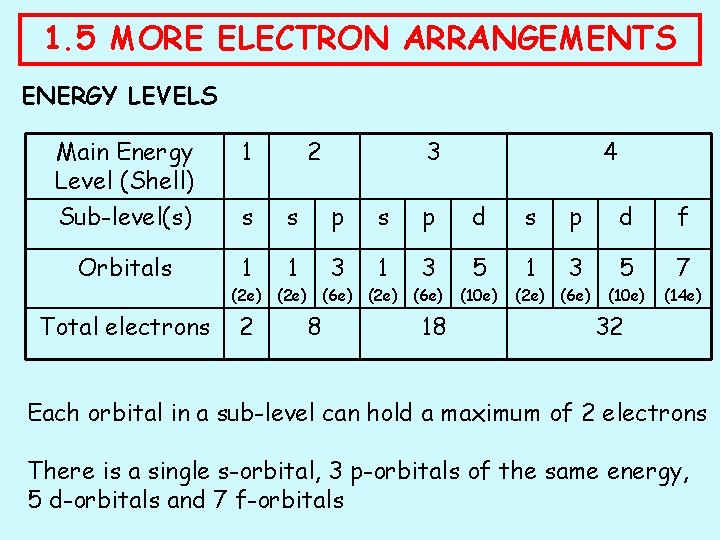
1. 5 MORE ELECTRON ARRANGEMENTS ENERGY LEVELS Main Energy Level (Shell) 1 2 3 Sub-level(s) s s p d f Orbitals 1 1 3 5 7 (10 e) (14 e) (2 e) (6 e) Total electrons 2 8 18 4 (10 e) (2 e) (6 e) 32 Each orbital in a sub-level can hold a maximum of 2 electrons There is a single s-orbital, 3 p-orbitals of the same energy, 5 d-orbitals and 7 f-orbitals
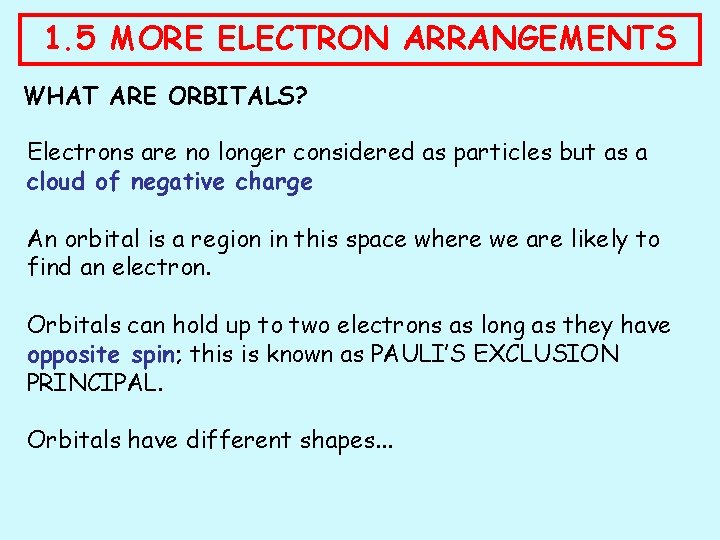
1. 5 MORE ELECTRON ARRANGEMENTS WHAT ARE ORBITALS? Electrons are no longer considered as particles but as a cloud of negative charge An orbital is a region in this space where we are likely to find an electron. Orbitals can hold up to two electrons as long as they have opposite spin; this is known as PAULI’S EXCLUSION PRINCIPAL. Orbitals have different shapes. . .
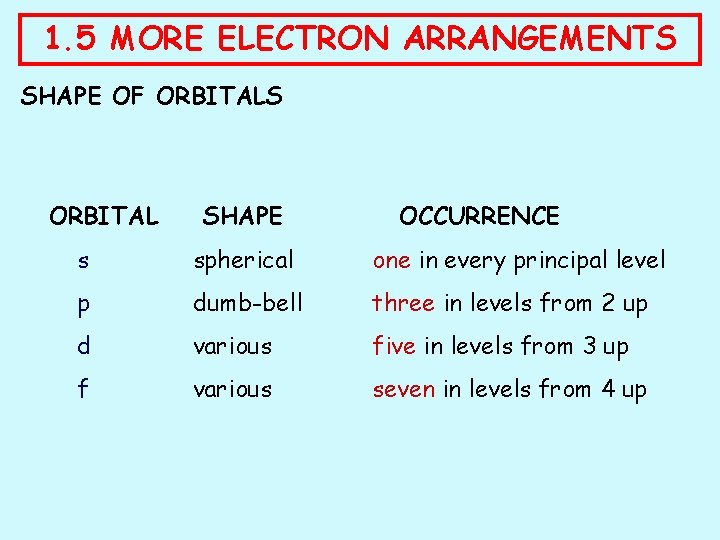
1. 5 MORE ELECTRON ARRANGEMENTS SHAPE OF ORBITALS ORBITAL SHAPE OCCURRENCE s spherical one in every principal level p dumb-bell three in levels from 2 up d various five in levels from 3 up f various seven in levels from 4 up
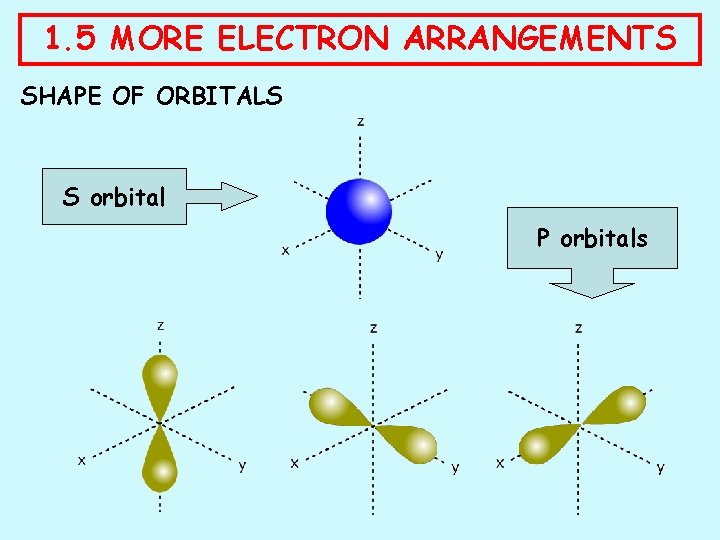
1. 5 MORE ELECTRON ARRANGEMENTS SHAPE OF ORBITALS S orbital P orbitals
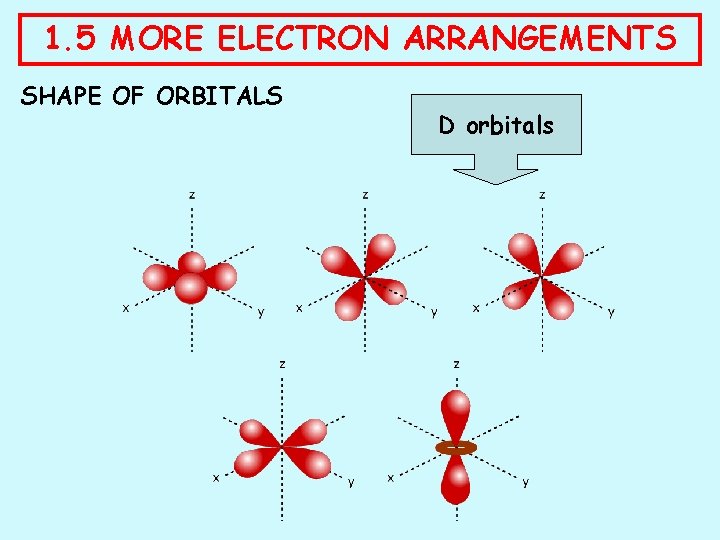
1. 5 MORE ELECTRON ARRANGEMENTS SHAPE OF ORBITALS D orbitals
- Slides: 50