A Hybrid Hydrogen Storage System Based on Hollow

A Hybrid Hydrogen Storage System Based on Hollow Glass Microspheres C. Eisenmennger-Sittner 1 and G. H. S. Drexler-Schmid 2, 1 Vienna University of Technology, Institute of Solid State Physics, Vienna, Austria 2 Austrian Institute of Technology, Vienna, Austria INTRODUCTION A COMBINED STORAGE SYSTEM COATING SMALL FRAGILE OBJECTS CATALYTIC REACTION AND REPEATED USE REQUIREMENTS FOR PRESSURIZATION CONCLUSION AND FUTURE ASPECTS VAASSCAA 9, Sydney, August 13 – 16, 2018
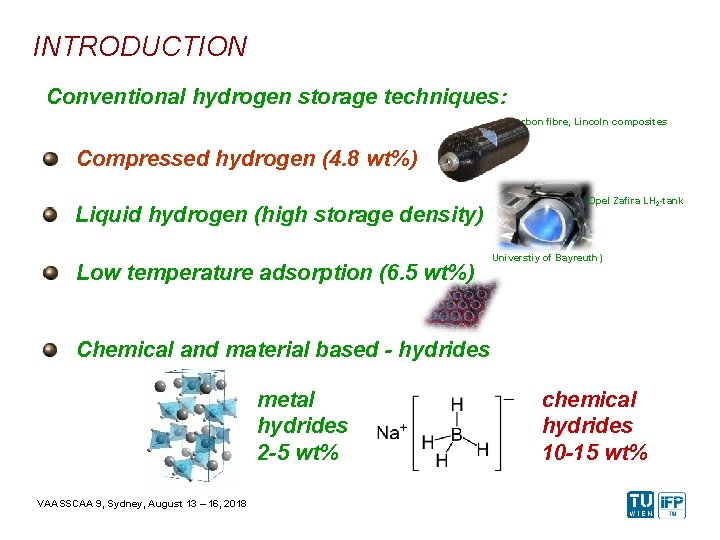
INTRODUCTION Conventional hydrogen storage techniques: Carbon fibre, Lincoln composites Compressed hydrogen (4. 8 wt%) Liquid hydrogen (high storage density) Low temperature adsorption (6. 5 wt%) Opel Zafira LH 2 -tank Universtiy of Bayreuth) Chemical and material based - hydrides metal hydrides 2 -5 wt% VAASSCAA 9, Sydney, August 13 – 16, 2018 chemical hydrides 10 -15 wt%
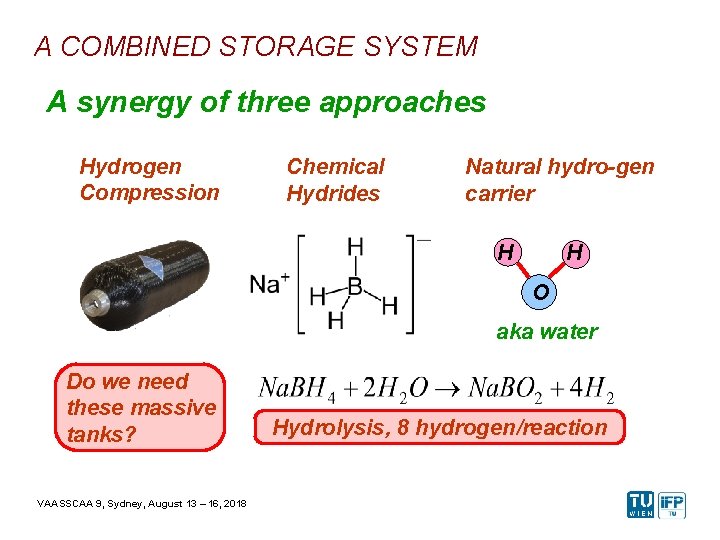
A COMBINED STORAGE SYSTEM A synergy of three approaches Hydrogen Compression Chemical Hydrides Natural hydro-gen carrier H H O aka water Do we need these massive tanks? VAASSCAA 9, Sydney, August 13 – 16, 2018 Hydrolysis, 8 hydrogen/reaction

THE AMAZING GLASS MICROSPHERES 1 µm 45 µm s = 6 GPa = 300 bar VAASSCAA 9, Sydney, August 13 – 16, 2018
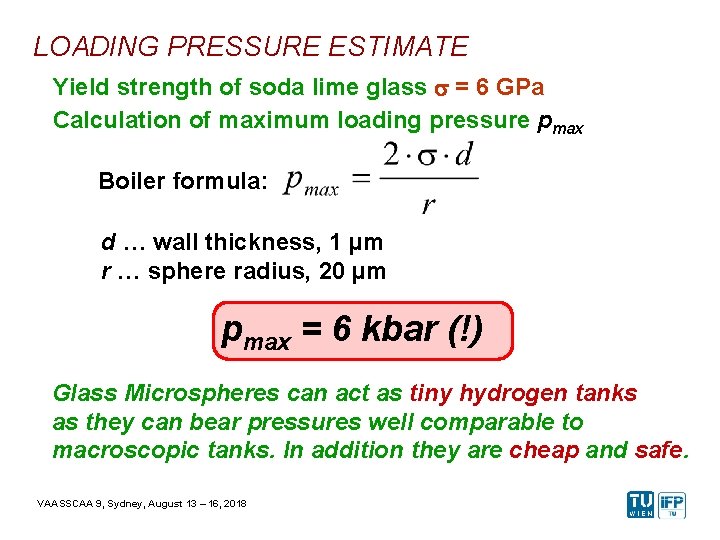
LOADING PRESSURE ESTIMATE Yield strength of soda lime glass s = 6 GPa Calculation of maximum loading pressure pmax Boiler formula: d … wall thickness, 1 µm r … sphere radius, 20 µm pmax = 6 kbar (!) Glass Microspheres can act as tiny hydrogen tanks as they can bear pressures well comparable to macroscopic tanks. In addition they are cheap and safe. VAASSCAA 9, Sydney, August 13 – 16, 2018

HYDROGEN LOADING Hydrogen can be LOADED into hollow glass microspheres by diffusion of H 2 into the sphere at high pressure (approx. 30 -70 MPa) and high temperature (approx. 200°C): H 2 high outside pressure (500 -700 bar) high outside temperature (200 °C) 45 µm VAASSCAA 9, Sydney, August 13 – 16, 2018
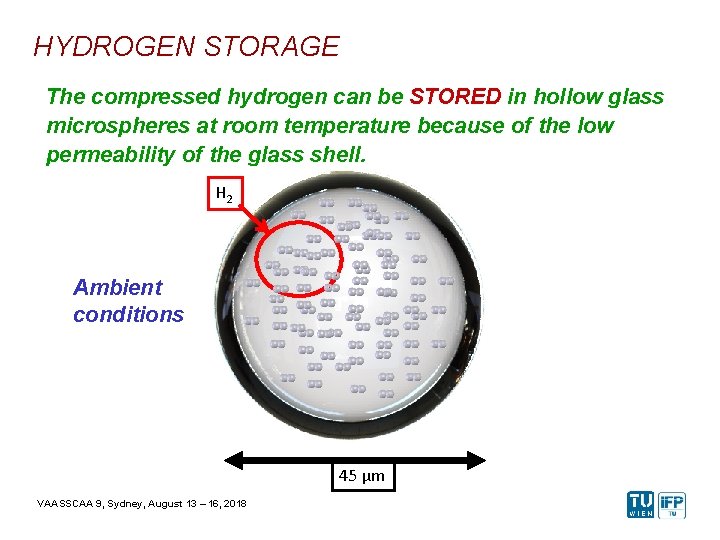
HYDROGEN STORAGE The compressed hydrogen can be STORED in hollow glass microspheres at room temperature because of the low permeability of the glass shell. H 2 Ambient conditions 45 µm VAASSCAA 9, Sydney, August 13 – 16, 2018

HYDROGEN RELEASE Hydrogen can be RELEASED from the microspheres by thermally activated diffusion at elevated temperature. 45 µm VAASSCAA 9, Sydney, August 13 – 16, 2018
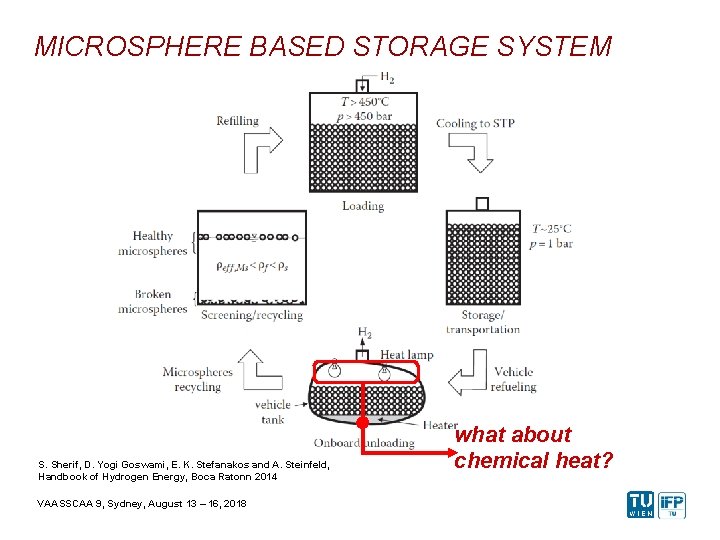
MICROSPHERE BASED STORAGE SYSTEM S. Sherif, D. Yogi Goswami, E. K. Stefanakos and A. Steinfeld, Handbook of Hydrogen Energy, Boca Ratonn 2014 VAASSCAA 9, Sydney, August 13 – 16, 2018 what about chemical heat?
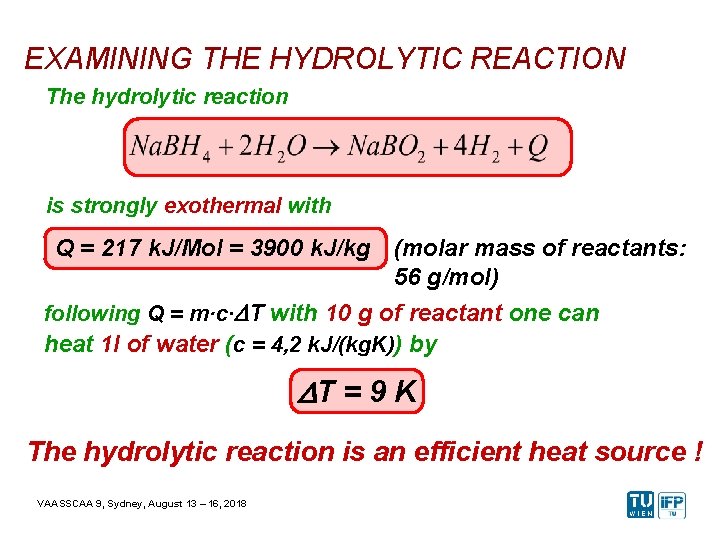
EXAMINING THE HYDROLYTIC REACTION The hydrolytic reaction is strongly exothermal with Q = 217 k. J/Mol = 3900 k. J/kg (molar mass of reactants: 56 g/mol) following Q = m·c·DT with 10 g of reactant one can heat 1 l of water (c = 4, 2 k. J/(kg. K)) by DT = 9 K The hydrolytic reaction is an efficient heat source ! VAASSCAA 9, Sydney, August 13 – 16, 2018
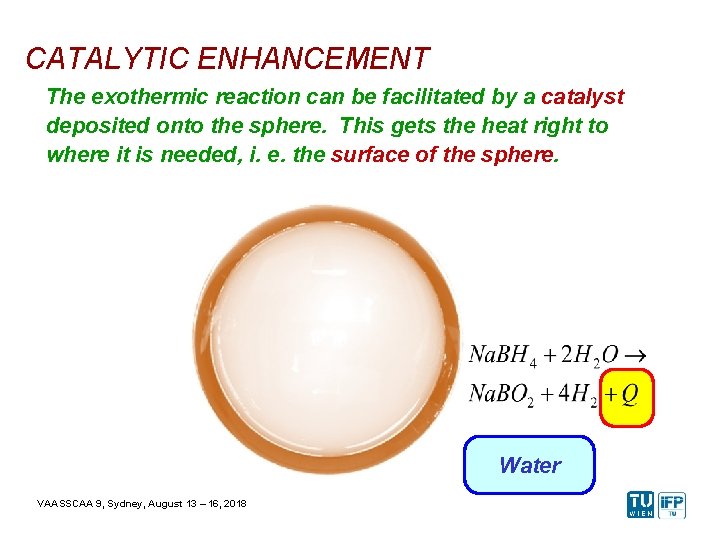
CATALYTIC ENHANCEMENT The exothermic reaction can be facilitated by a catalyst deposited onto the sphere. This gets the heat right to where it is needed, i. e. the surface of the sphere. Catalyst Water VAASSCAA 9, Sydney, August 13 – 16, 2018
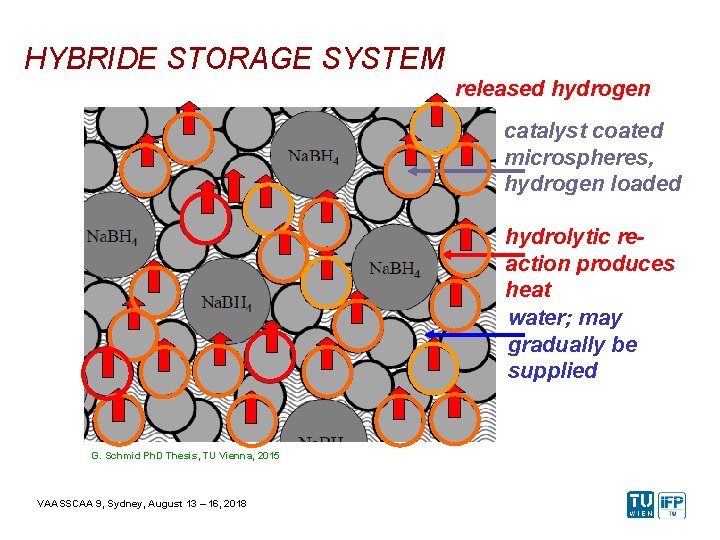
HYBRIDE STORAGE SYSTEM released hydrogen catalyst coated microspheres, hydrogen loaded hydrolytic reaction produces heat water; may gradually be supplied G. Schmid Ph. D Thesis, TU Vienna, 2015 VAASSCAA 9, Sydney, August 13 – 16, 2018
ITEMS NEEDED Glass microspheres Catalyst material Coating method ü ü ü Evaluation of efficiency of hydrolysis Pressurization of coated spheres ü D Evaluation of total system performance VAASSCAA 9, Sydney, August 13 – 16, 2018 D
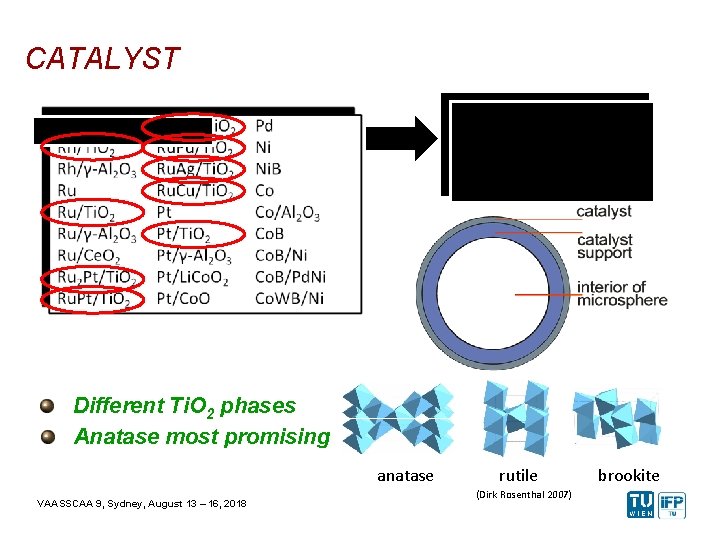
CATALYST Chosen Catalyst Metals (oxide support) Pt or Ru noble metal Ti. O 2 as support material Different Ti. O 2 phases Anatase most promising anatase VAASSCAA 9, Sydney, August 13 – 16, 2018 rutile (Dirk Rosenthal 2007) brookite
COATING METHOD Platinum, Ruthenium Inert, high melting point metals Titanium Oxides Stable, high melting point oxides Requirement posed by substrate Relatively low deposition temperature, smaller than softening point of borosilicte glass (approx. 400°C) Particle containment and intermixing necessary Non-reactive or reactive magnetron sputtering as process of choice VAASSCAA 9, Sydney, August 13 – 16, 2018
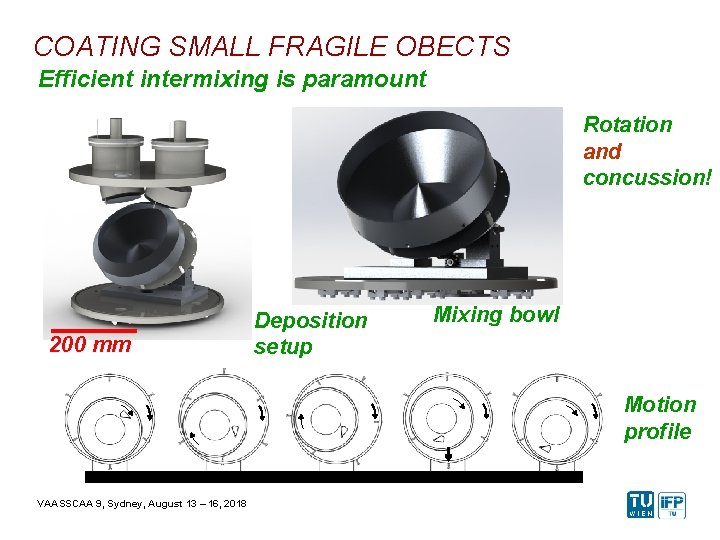
COATING SMALL FRAGILE OBECTS Efficient intermixing is paramount Rotation and concussion! 200 mm Deposition setup Mixing bowl Motion profile VAASSCAA 9, Sydney, August 13 – 16, 2018

COATED SPHERES uncoated target 100 µm coated with catalyst 45° VAASSCAA 9, Sydney, August 13 – 16, 2018
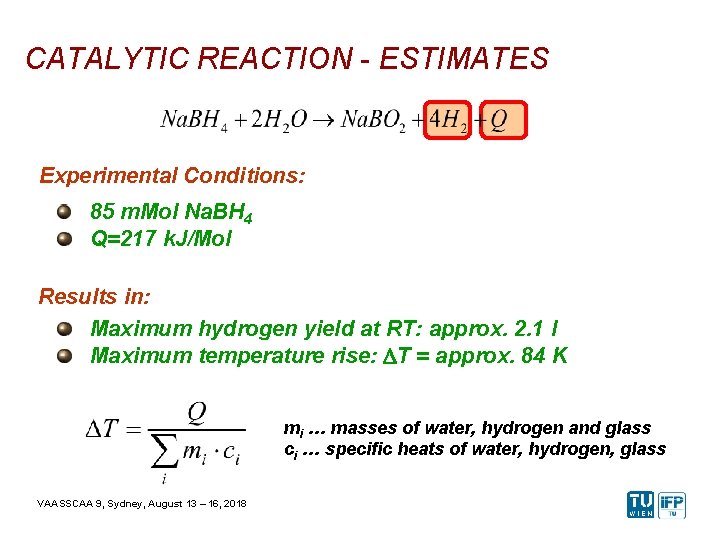
CATALYTIC REACTION - ESTIMATES Experimental Conditions: 85 m. Mol Na. BH 4 Q=217 k. J/Mol Results in: Maximum hydrogen yield at RT: approx. 2. 1 l Maximum temperature rise: DT = approx. 84 K mi … masses of water, hydrogen and glass ci … specific heats of water, hydrogen, glass VAASSCAA 9, Sydney, August 13 – 16, 2018
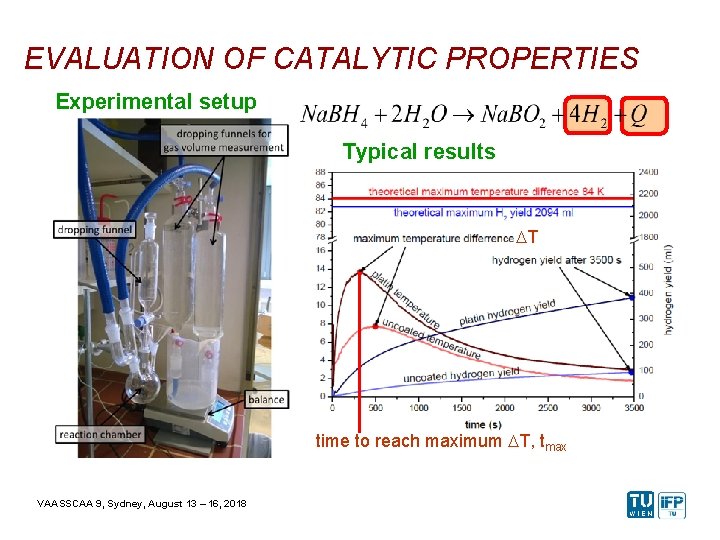
EVALUATION OF CATALYTIC PROPERTIES Experimental setup Typical results DT time to reach maximum DT, tmax VAASSCAA 9, Sydney, August 13 – 16, 2018
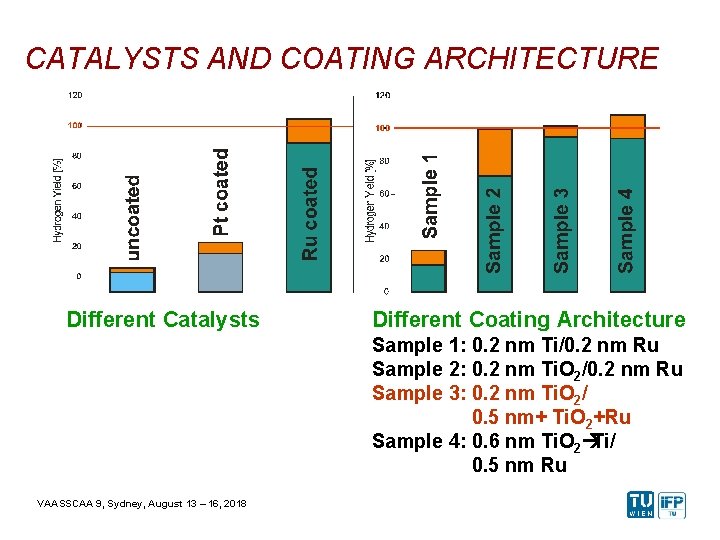
CATALYSTS AND COATING ARCHITECTURE Different Catalysts Different Coating Architecture Sample 1: 0. 2 nm Ti/0. 2 nm Ru Sample 2: 0. 2 nm Ti. O 2/0. 2 nm Ru Sample 3: 0. 2 nm Ti. O 2/ 0. 5 nm+ Ti. O 2+Ru Sample 4: 0. 6 nm Ti. O 2àTi/ 0. 5 nm Ru VAASSCAA 9, Sydney, August 13 – 16, 2018
REPEATED USE – ACTIVATION PROCEDURE Take spheres from concluded catalysis experiment. Start reactivation procedure as soon as possible. Rinse spheres in distilled water. Perform at least two rinsing runs. Set p. H value, which is alcaline after catalysis, to neutral by rinsing in diluted hydrochloric acid. Rinse again in distilled watrwe ro get rid of acidic residues. VAASSCAA 9, Sydney, August 13 – 16, 2018
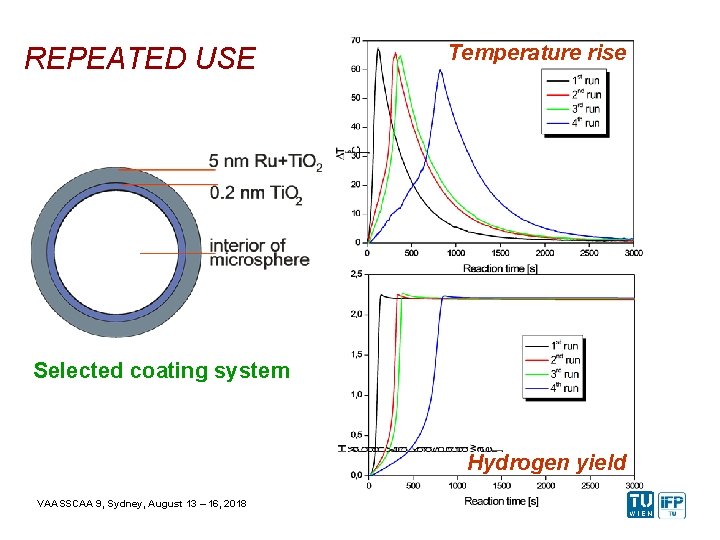
REPEATED USE Temperature rise Selected coating system Hydrogen yield VAASSCAA 9, Sydney, August 13 – 16, 2018
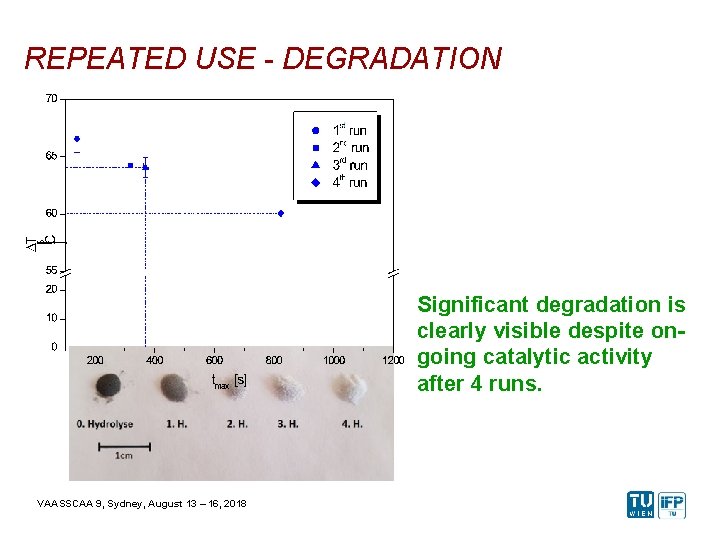
REPEATED USE - DEGRADATION Significant degradation is clearly visible despite ongoing catalytic activity after 4 runs. VAASSCAA 9, Sydney, August 13 – 16, 2018
REQUIREMENTS FOR PRESSURIZATION Hydrogen pressure and external temperature Microspheres should be pressurized at pressures as high as possible, i. e. approx. 600 bar. Filling temperatures should ensure rapid permeation without glass softening, i. e. approx. 300°C. Coating system stability The system of adhesion promoter/catalyst support has to withstand the pressurization conditions and has to leave the permeability of the spheres unaltered. This has not been checked yet! VAASSCAA 9, Sydney, August 13 – 16, 2018
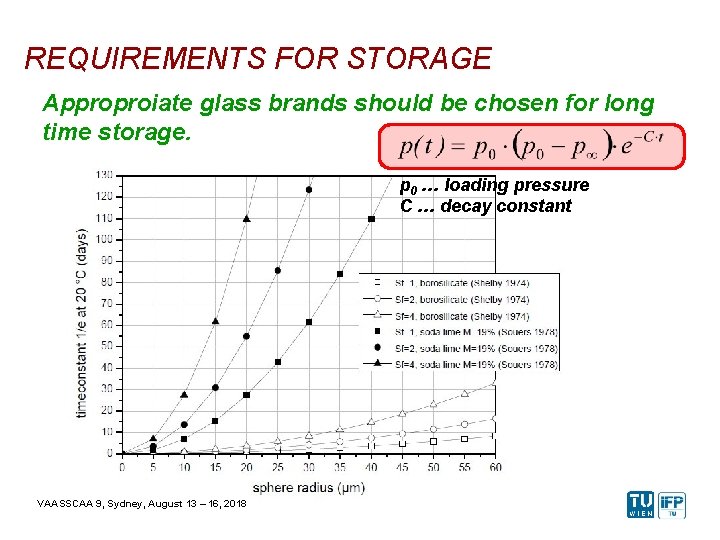
REQUIREMENTS FOR STORAGE Approproiate glass brands should be chosen for long time storage. p 0 … loading pressure C … decay constant VAASSCAA 9, Sydney, August 13 – 16, 2018
CONCLUSION AND FUTURE ASPECTS Basics of a hybride H-storage system The basic ingredients of a hybride hydrogen storage system combining chemical storage and storage in pressurized glass microspheres could be manufactured. Catalytic performance The theoretical limits of the hydrolytic reaction of sodium borohydride with water could be reached in respect to hydrogen yield and reaction temperture. Total system performance The total system performace has to be tested on pressurized coated microspheres. The influence of the coating on hydrogen loading and release has to be evaluated. VAASSCAA 9, Sydney, August 13 – 16, 2018
ACKNOWLEDGEMENTS This work was financially supported by the Austrian Science Fund, Grants P-19379, P-22718, TRP-6 and TRP-281 VAASSCAA 9, Sydney, August 13 – 16, 2018
- Slides: 27