A HitchHikers Guide to Molecular Thermodynamics What really



- Slides: 12

A Hitch-Hiker’s Guide to Molecular Thermodynamics What really makes proteins fold and ligands bind Alan Cooper Chemistry Department Joseph Black Building, Glasgow University Glasgow G 12 8 QQ, Scotland Amsterdam: November 2002

“Concepts and tools for medicinal chemists” + What makes this protein fold, and what controls its stability ?

“Concepts and tools for medicinal chemists” + What makes this protein fold, and what controls its stability ? What are thermodynamic forces responsible for ligand binding ? Can we use them to design better ligands ?

“ Concepts and tools for medicinal chemists” Thermodynamic homeostasis, compensation; hydrogen-bonded lattices…. . . the role of water in biomolecular interactions Microcalorimetry: analytical uses for biomolecular interactions and stability

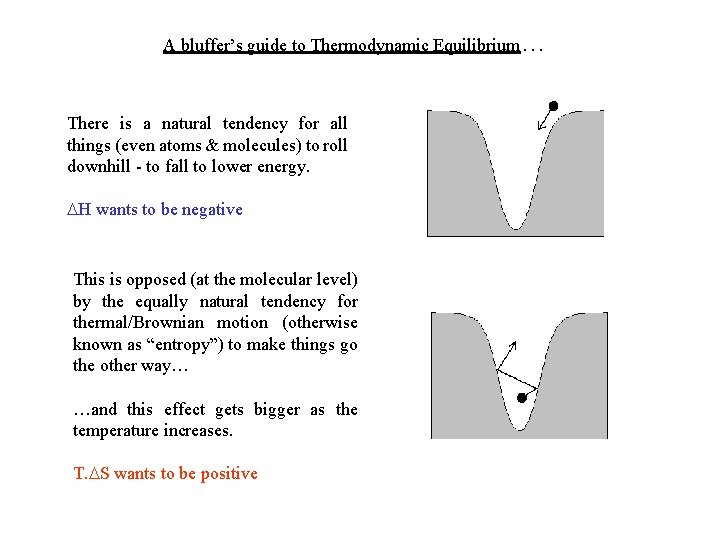
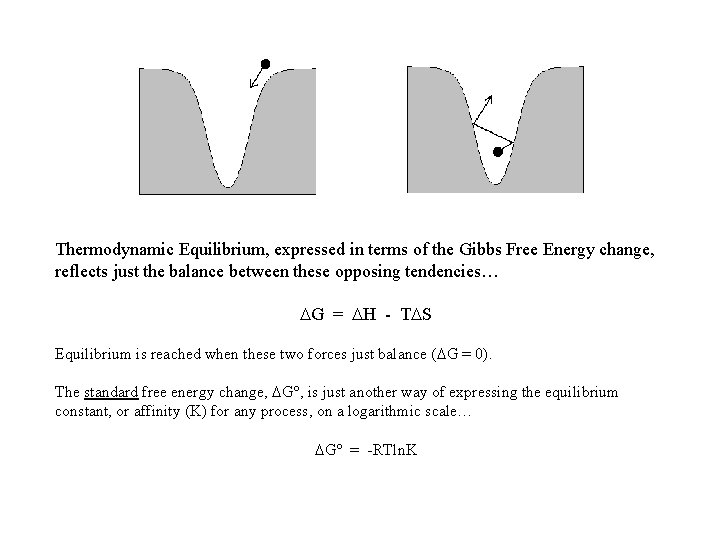
A bluffer’s guide to Thermodynamic Equilibrium… There is a natural tendency for all things (even atoms & molecules) to roll downhill - to fall to lower energy. H wants to be negative This is opposed (at the molecular level) by the equally natural tendency for thermal/Brownian motion (otherwise known as “entropy”) to make things go the other way… …and this effect gets bigger as the temperature increases. T. S wants to be positive

Thermodynamic Equilibrium, expressed in terms of the Gibbs Free Energy change, reflects just the balance between these opposing tendencies… G = H - T S Equilibrium is reached when these two forces just balance ( G = 0). The standard free energy change, G , is just another way of expressing the equilibrium constant, or affinity (K) for any process, on a logarithmic scale… G = -RTln. K

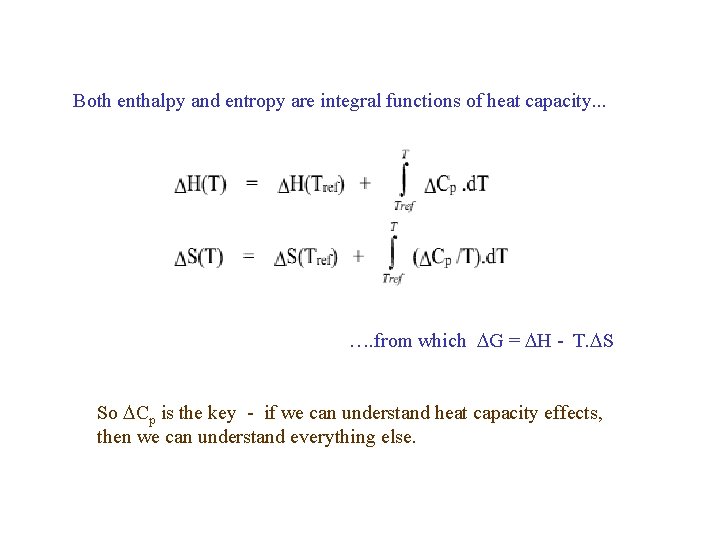
Both enthalpy and entropy are integral functions of heat capacity. . . …. from which G = H - T. S So Cp is the key - if we can understand heat capacity effects, then we can understand everything else.

Calorimetric techniques. . . • Differential scanning calorimetry (DSC) • Isothermal titration calorimetry (ITC) • Pressure perturbation calorimetry (PPC)

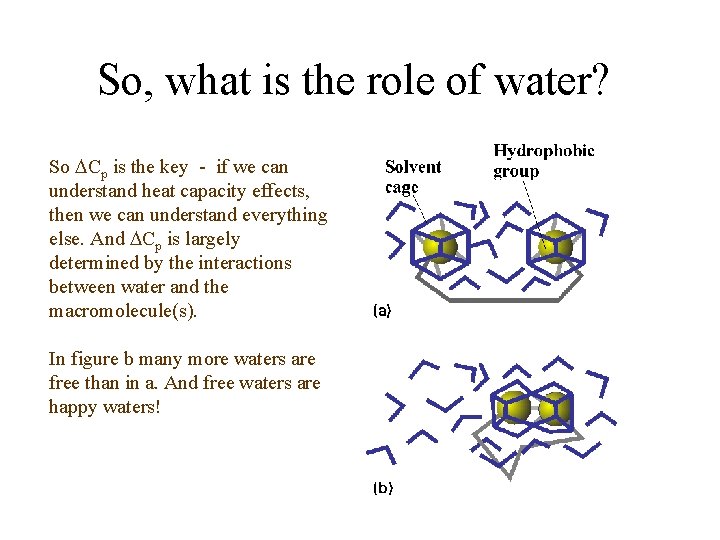
So, what is the role of water? So Cp is the key - if we can understand heat capacity effects, then we can understand everything else. And Cp is largely determined by the interactions between water and the macromolecule(s). In figure b many more waters are free than in a. And free waters are happy waters!

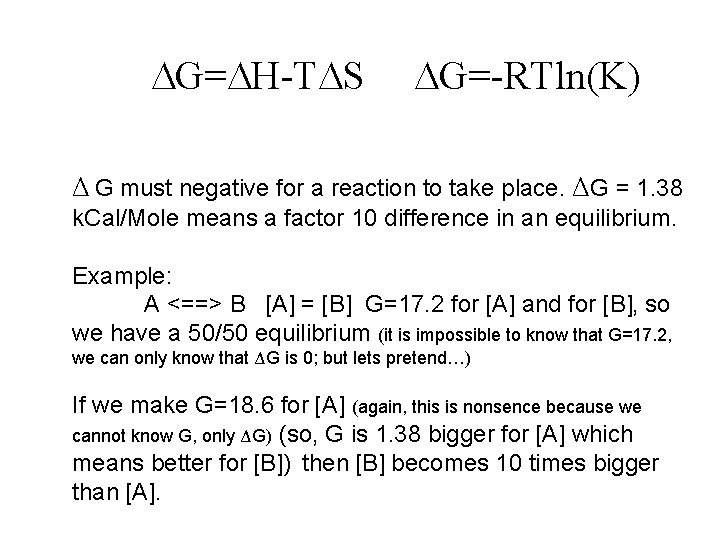
G= H-T S G=-RTln(K) Δ G must negative for a reaction to take place. ΔG = 1. 38 k. Cal/Mole means a factor 10 difference in an equilibrium. Example: A <==> B [A] = [B] G=17. 2 for [A] and for [B], so we have a 50/50 equilibrium (it is impossible to know that G=17. 2, we can only know that ΔG is 0; but lets pretend…) If we make G=18. 6 for [A] (again, this is nonsence because we cannot know G, only ΔG) (so, G is 1. 38 bigger for [A] which means better for [B]) then [B] becomes 10 times bigger than [A].

G= H-T S Good for Δ H: 1) Contacts in protein (H-bonds, Van der Waals interactions, salt bridges, aromatic stacking, etc). 2) H-bonds between water molecules Bad for Δ H: 1) H-bonds between water and part of protein that gets buried.

G= H-T S Good for Δ S: Entropy of water. Bad for Δ S: Entropy of protein.