A high throughput cell culture platform for bioprocess

A high throughput cell culture platform for bioprocess optimization Seth Rodgers, CTO Bioprocessors CPAC Rome March 20, 2007 1

Outline • The role of model systems in process understanding • A scale-down model bioreactor and the data sets we get now • Challenges remaining – especially the data sets we’d like to get © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 2
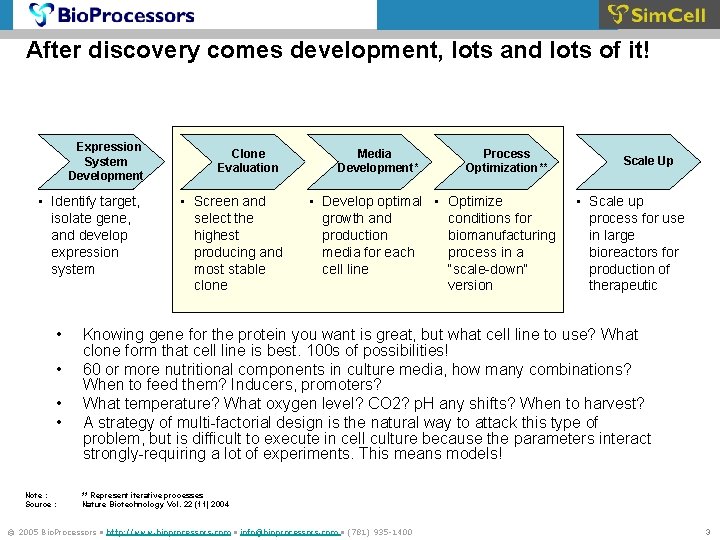
After discovery comes development, lots and lots of it! Expression System Development • Identify target, isolate gene, and develop expression system • • Note : Source : Clone Evaluation • Screen and select the highest producing and most stable clone Media Flasks Development* Process Optimization** • Develop optimal • Optimize conditions for growth and biomanufacturing production process in a media for each “scale-down” cell line version Scale Up • Scale up process for use in large bioreactors for production of therapeutic Knowing gene for the protein you want is great, but what cell line to use? What clone form that cell line is best. 100 s of possibilities! 60 or more nutritional components in culture media, how many combinations? When to feed them? Inducers, promoters? What temperature? What oxygen level? CO 2? p. H any shifts? When to harvest? A strategy of multi-factorial design is the natural way to attack this type of problem, but is difficult to execute in cell culture because the parameters interact strongly-requiring a lot of experiments. This means models! ** Represent iterative processes Nature Biotechnology Vol. 22 (11) 2004 © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 3
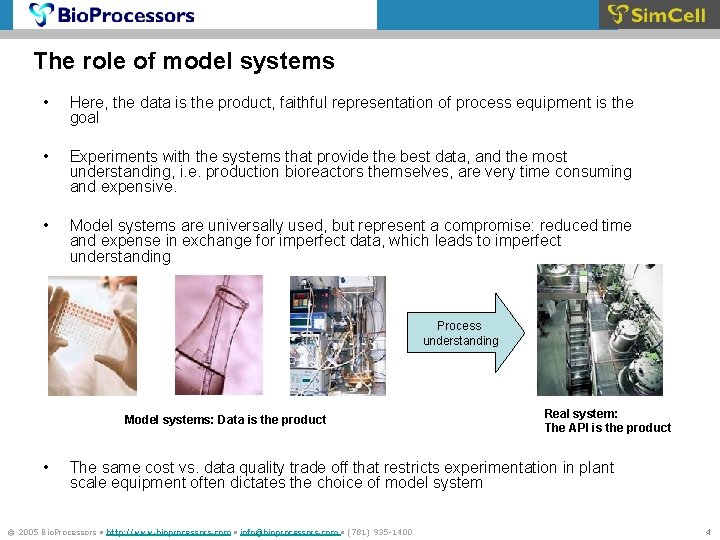
The role of model systems • Here, the data is the product, faithful representation of process equipment is the goal • Experiments with the systems that provide the best data, and the most understanding, i. e. production bioreactors themselves, are very time consuming and expensive. • Model systems are universally used, but represent a compromise: reduced time and expense in exchange for imperfect data, which leads to imperfect understanding Process understanding Model systems: Data is the product • Real system: The API is the product The same cost vs. data quality trade off that restricts experimentation in plant scale equipment often dictates the choice of model system © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 4
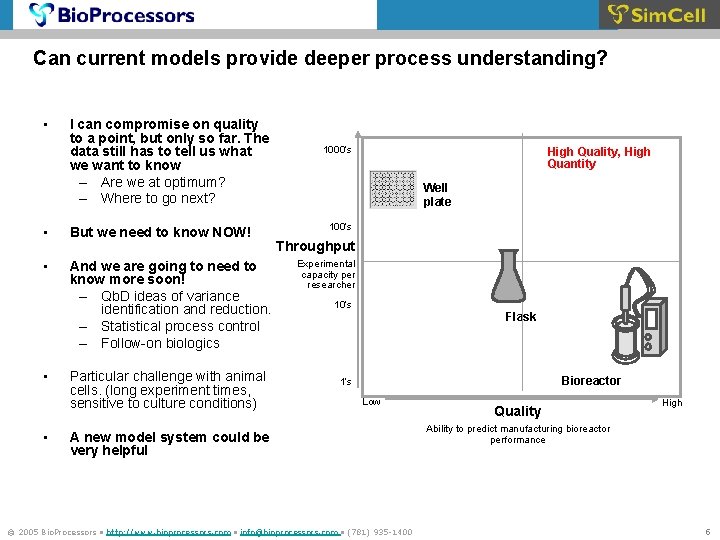
Can current models provide deeper process understanding? • I can compromise on quality to a point, but only so far. The data still has to tell us what we want to know – Are we at optimum? – Where to go next? • But we need to know NOW! • And we are going to need to know more soon! – Qb. D ideas of variance identification and reduction. – Statistical process control – Follow-on biologics • • Particular challenge with animal cells. (long experiment times, sensitive to culture conditions) 1000’s High Quality, High Quantity Well plate 100’s Throughput Experimental capacity per researcher 10’s Flask Bioreactor 1’s Low A new model system could be very helpful © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 Quality High Ability to predict manufacturing bioreactor performance 5
Some High-Throughput Cell Culture System Requirements • • • Deliver meaningful scalable data Sustain cells, control temperature, O 2, CO 2, p. H, agitation Maintain sterility Monitor cell density, p. H, DO, metabolites, product titer Operate with accuracy and precision and provide control of process parameters comparable to bench top bioreactor systems • Automatic operation with minimal operator supervision • Integration with tools for designing experiments and handling data © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 6
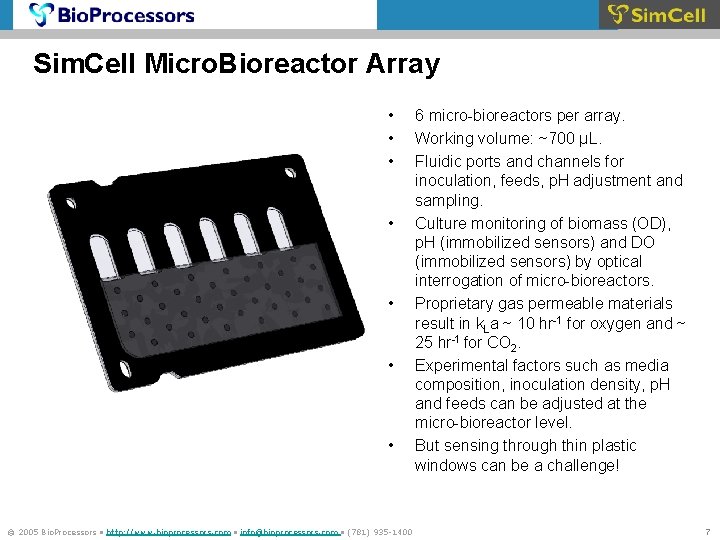
Sim. Cell Micro. Bioreactor Array • • © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 6 micro-bioreactors per array. Working volume: ~700 µL. Fluidic ports and channels for inoculation, feeds, p. H adjustment and sampling. Culture monitoring of biomass (OD), p. H (immobilized sensors) and DO (immobilized sensors) by optical interrogation of micro-bioreactors. Proprietary gas permeable materials result in k. La ~ 10 hr-1 for oxygen and ~ 25 hr-1 for CO 2. Experimental factors such as media composition, inoculation density, p. H and feeds can be adjusted at the micro-bioreactor level. But sensing through thin plastic windows can be a challenge! 7
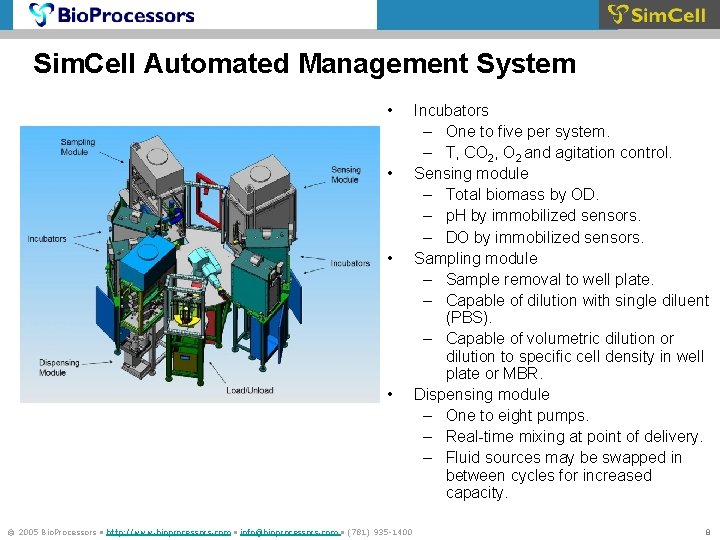
Sim. Cell Automated Management System • • © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 Incubators – One to five per system. – T, CO 2, O 2 and agitation control. Sensing module – Total biomass by OD. – p. H by immobilized sensors. – DO by immobilized sensors. Sampling module – Sample removal to well plate. – Capable of dilution with single diluent (PBS). – Capable of volumetric dilution or dilution to specific cell density in well plate or MBR. Dispensing module – One to eight pumps. – Real-time mixing at point of delivery. – Fluid sources may be swapped in between cycles for increased capacity. 8

Sim. Cell Automated Management System (SAMS) © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 9
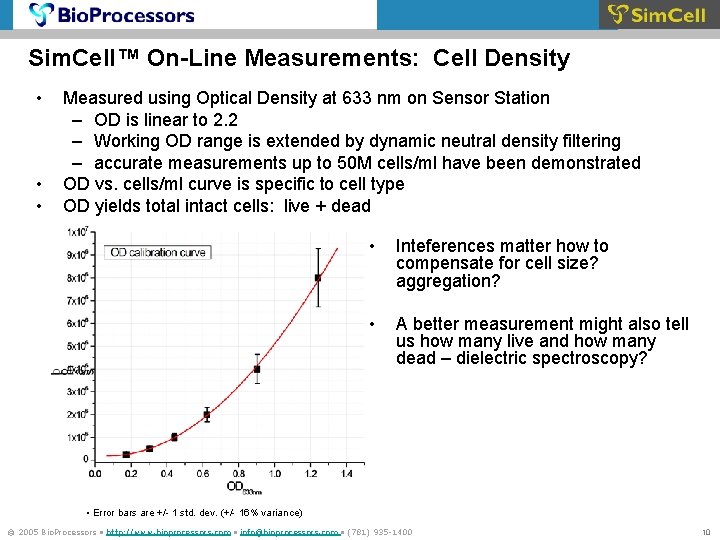
Sim. Cell™ On-Line Measurements: Cell Density • • • Measured using Optical Density at 633 nm on Sensor Station – OD is linear to 2. 2 – Working OD range is extended by dynamic neutral density filtering – accurate measurements up to 50 M cells/ml have been demonstrated OD vs. cells/ml curve is specific to cell type OD yields total intact cells: live + dead • Inteferences matter how to compensate for cell size? aggregation? • A better measurement might also tell us how many live and how many dead – dielectric spectroscopy? • Error bars are +/- 1 std. dev. (+/- 16% variance) © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 10
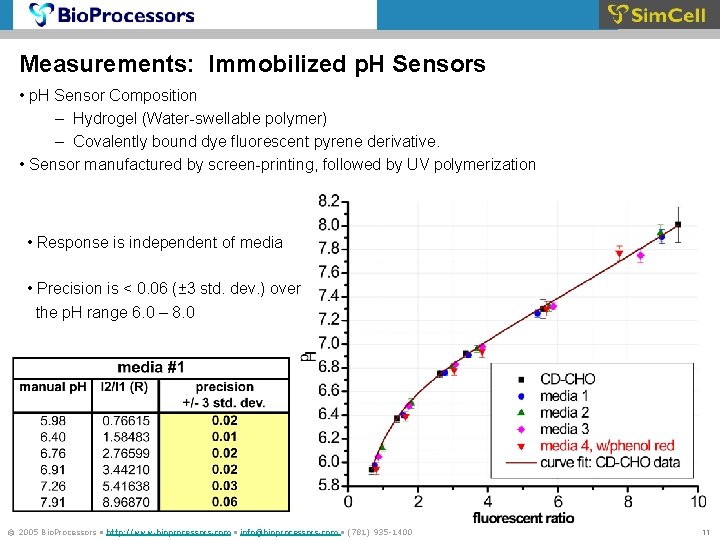
Measurements: Immobilized p. H Sensors • p. H Sensor Composition – Hydrogel (Water-swellable polymer) – Covalently bound dye fluorescent pyrene derivative. • Sensor manufactured by screen-printing, followed by UV polymerization • Response is independent of media • Precision is < 0. 06 (± 3 std. dev. ) over the p. H range 6. 0 – 8. 0 © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 11
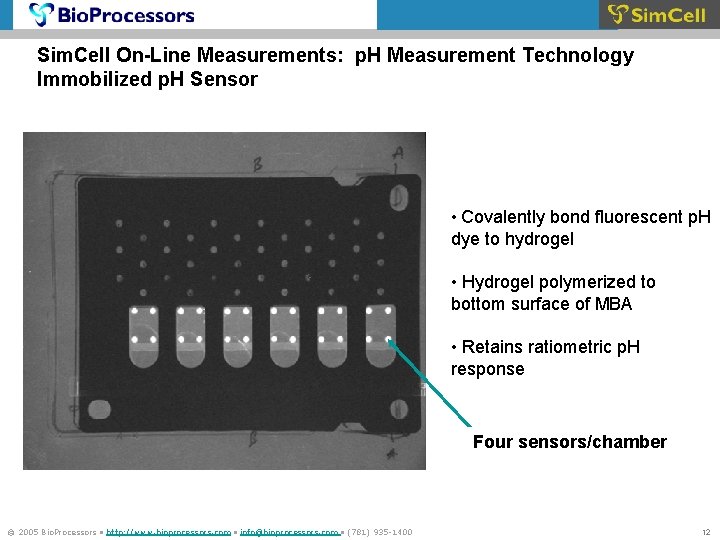
Sim. Cell On-Line Measurements: p. H Measurement Technology Immobilized p. H Sensor • Covalently bond fluorescent p. H dye to hydrogel • Hydrogel polymerized to bottom surface of MBA • Retains ratiometric p. H response Four sensors/chamber © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 12
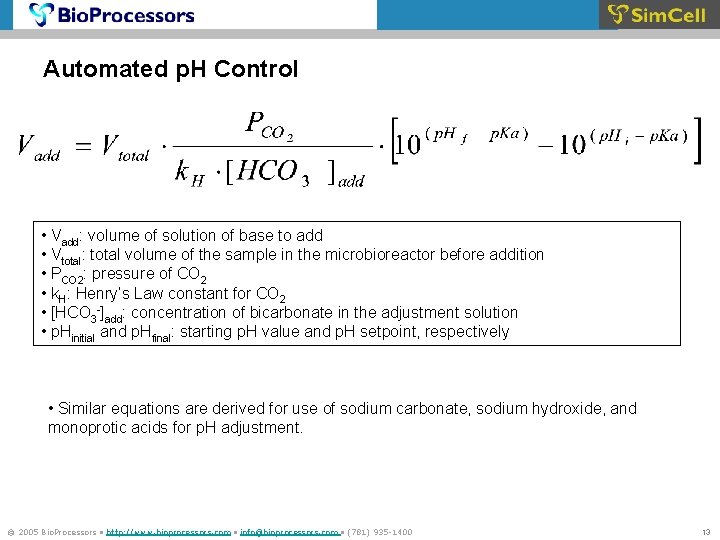
Automated p. H Control • Vadd: volume of solution of base to add • Vtotal: total volume of the sample in the microbioreactor before addition • PCO 2: pressure of CO 2 • k. H: Henry’s Law constant for CO 2 • [HCO 3 -]add: concentration of bicarbonate in the adjustment solution • p. Hinitial and p. Hfinal: starting p. H value and p. H setpoint, respectively • Similar equations are derived for use of sodium carbonate, sodium hydroxide, and monoprotic acids for p. H adjustment. © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 13
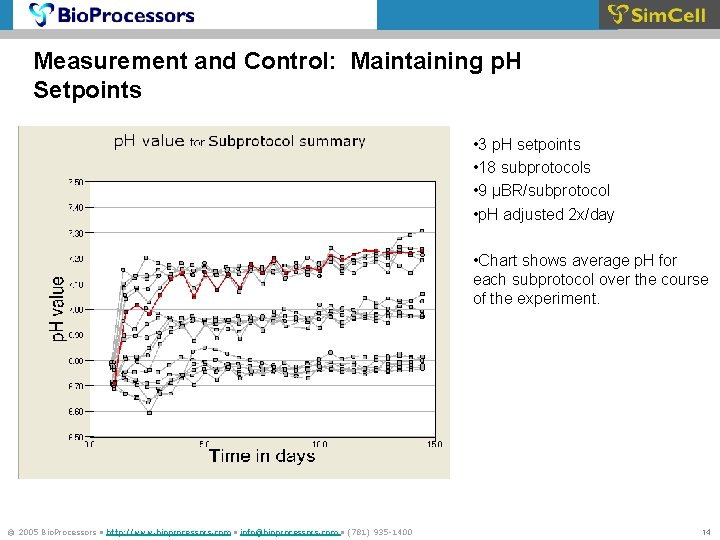
Measurement and Control: Maintaining p. H Setpoints • 3 p. H setpoints • 18 subprotocols • 9 μBR/subprotocol • p. H adjusted 2 x/day • Chart shows average p. H for each subprotocol over the course of the experiment. © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 14
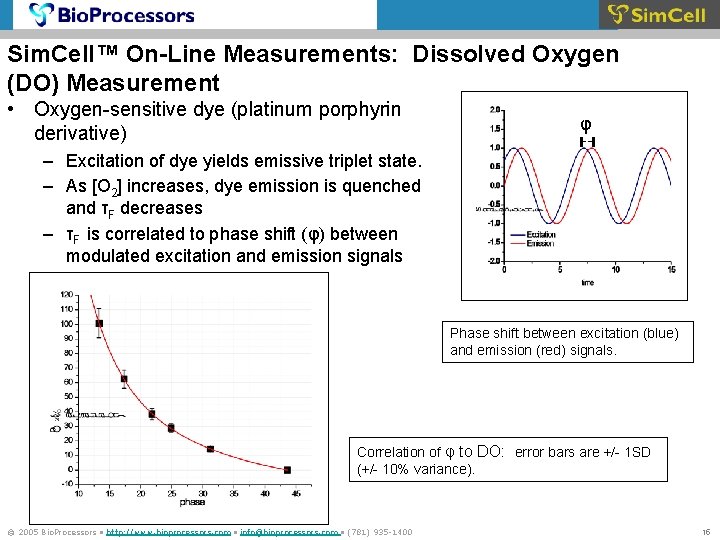
Sim. Cell™ On-Line Measurements: Dissolved Oxygen (DO) Measurement • Oxygen-sensitive dye (platinum porphyrin derivative) φ – Excitation of dye yields emissive triplet state. – As [O 2] increases, dye emission is quenched and τF decreases – τF is correlated to phase shift (φ) between modulated excitation and emission signals Phase shift between excitation (blue) and emission (red) signals. Correlation of φ to DO: error bars are +/- 1 SD (+/- 10% variance). © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 15
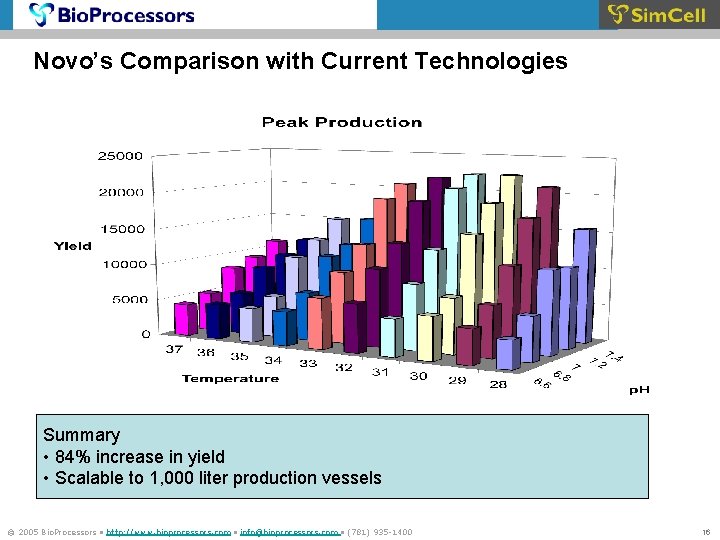
Novo’s Comparison with Current Technologies Summary • 84% increase in yield • Scalable to 1, 000 liter production vessels Significant improvement n process yield at lower cost and shorter time © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 16
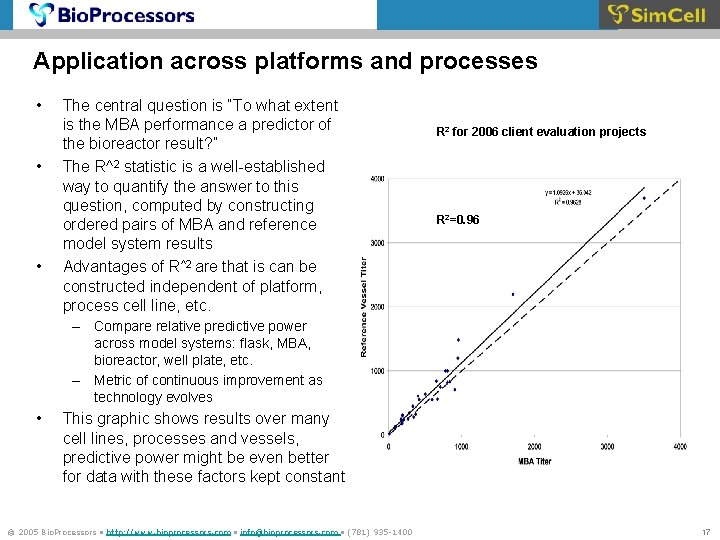
Application across platforms and processes • • • The central question is “To what extent is the MBA performance a predictor of the bioreactor result? ” The R^2 statistic is a well-established way to quantify the answer to this question, computed by constructing ordered pairs of MBA and reference model system results Advantages of R^2 are that is can be constructed independent of platform, process cell line, etc. R 2 for 2006 client evaluation projects R 2=0. 96 – Compare relative predictive power across model systems: flask, MBA, bioreactor, well plate, etc. – Metric of continuous improvement as technology evolves • This graphic shows results over many cell lines, processes and vessels, predictive power might be even better for data with these factors kept constant © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 17
What’s missing • Protein titer of course! – Enzymatic like ELISA is the most common, but it takes work, even with automation – Something else? • • • Viability Some understanding of the protein quality (glycosylation, aggregation) All those media components in the culture broth – Nutrients: glucose, glutamine, amino acids, vitamins – Metabolic products: lactate, etc. • • Can spectroscopy (NIR, MIR, Raman work here? ) Anything else useful in characterizing and ‘fingerprinting’ the process, that is, a useful predictor of process outcomes. • Ideal measurement (for us at least) is – Non invasive – it it needs a sample, best case is • • – – Small sample Works with crude broth, no pre- treatment Matched throughput Calibrated less frequently than once per MBA Compatible with flexible! plastic cell culture device (challenge for some spectroscopy) Cost competitive pulling samples and using well plates © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 18

Conclusions • Model systems are indispensable tools, and increasing demands for data will be difficult to meet with current platforms. • A high-throughput cell culture system presents a possible solution if the data is of sufficient quality to predict process outcomes. • Bio. Processors Sim. Cell system represents one possible solution that combines high throughput with highly representative data. © 2005 Bio. Processors • http: //www. bioprocessors. com • info@bioprocessors. com • (781) 935 -1400 19
- Slides: 19