A group of students are heating magnesium In

- Slides: 4

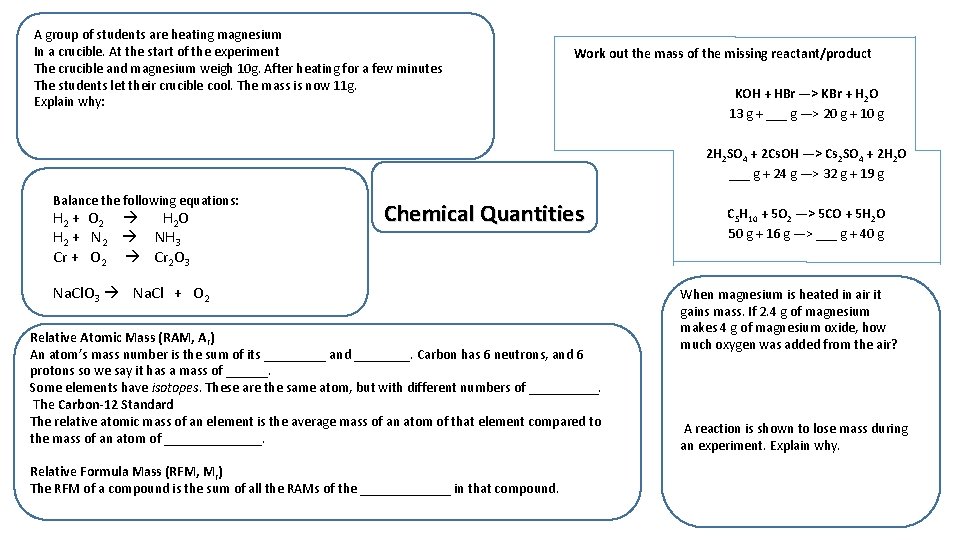
A group of students are heating magnesium In a crucible. At the start of the experiment The crucible and magnesium weigh 10 g. After heating for a few minutes The students let their crucible cool. The mass is now 11 g. Explain why: Work out the mass of the missing reactant/product KOH + HBr —> KBr + H 2 O 13 g + ___ g —> 20 g + 10 g 2 H 2 SO 4 + 2 Cs. OH —> Cs 2 SO 4 + 2 H 2 O ___ g + 24 g —> 32 g + 19 g Balance the following equations: H 2 + O 2 H 2 O H 2 + N 2 NH 3 Cr + O 2 Cr 2 O 3 Chemical Quantities Na. Cl. O 3 Na. Cl + O 2 Relative Atomic Mass (RAM, Ar) An atom’s mass number is the sum of its _____ and ____. Carbon has 6 neutrons, and 6 protons so we say it has a mass of ______. Some elements have isotopes. These are the same atom, but with different numbers of _____. The Carbon-12 Standard The relative atomic mass of an element is the average mass of an atom of that element compared to the mass of an atom of _______. Relative Formula Mass (RFM, Mr) The RFM of a compound is the sum of all the RAMs of the _______ in that compound. C 5 H 10 + 5 O 2 —> 5 CO + 5 H 2 O 50 g + 16 g —> ___ g + 40 g When magnesium is heated in air it gains mass. If 2. 4 g of magnesium makes 4 g of magnesium oxide, how much oxygen was added from the air? A reaction is shown to lose mass during an experiment. Explain why.

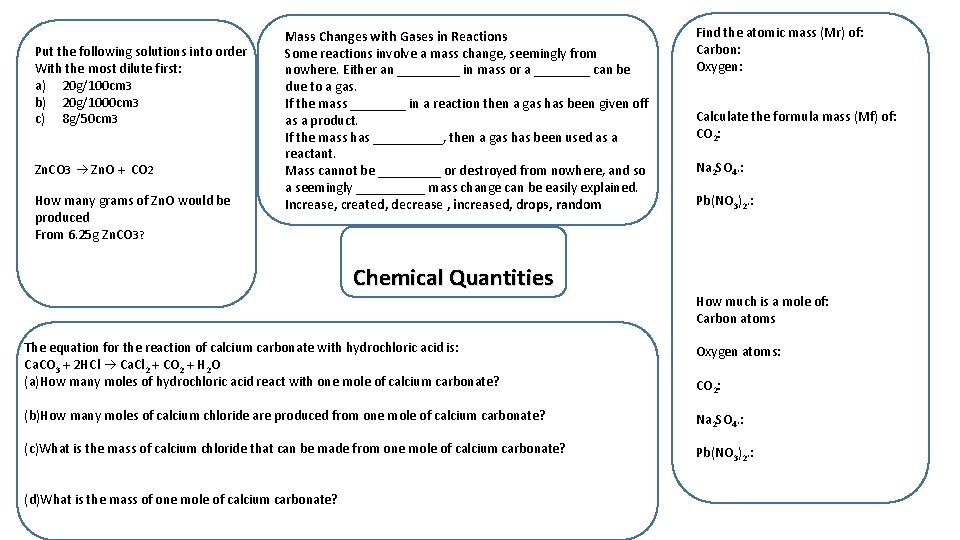
Put the following solutions into order With the most dilute first: a) 20 g/100 cm 3 b) 20 g/1000 cm 3 c) 8 g/50 cm 3 Zn. CO 3 Zn. O + CO 2 How many grams of Zn. O would be produced From 6. 25 g Zn. CO 3? Mass Changes with Gases in Reactions Some reactions involve a mass change, seemingly from nowhere. Either an _____ in mass or a ____ can be due to a gas. If the mass ____ in a reaction then a gas has been given off as a product. If the mass has _____, then a gas has been used as a reactant. Mass cannot be _____ or destroyed from nowhere, and so a seemingly _____ mass change can be easily explained. Increase, created, decrease , increased, drops, random Chemical Quantities Find the atomic mass (Mr) of: Carbon: Oxygen: Calculate the formula mass (Mf) of: CO 2: Na 2 SO 4. : Pb(NO 3)2. : How much is a mole of: Carbon atoms The equation for the reaction of calcium carbonate with hydrochloric acid is: Ca. CO 3 + 2 HCl Ca. Cl 2 + CO 2 + H 2 O (a)How many moles of hydrochloric acid react with one mole of calcium carbonate? Oxygen atoms: (b)How many moles of calcium chloride are produced from one mole of calcium carbonate? Na 2 SO 4. : (c)What is the mass of calcium chloride that can be made from one mole of calcium carbonate? Pb(NO 3)2. : (d)What is the mass of one mole of calcium carbonate? CO 2:

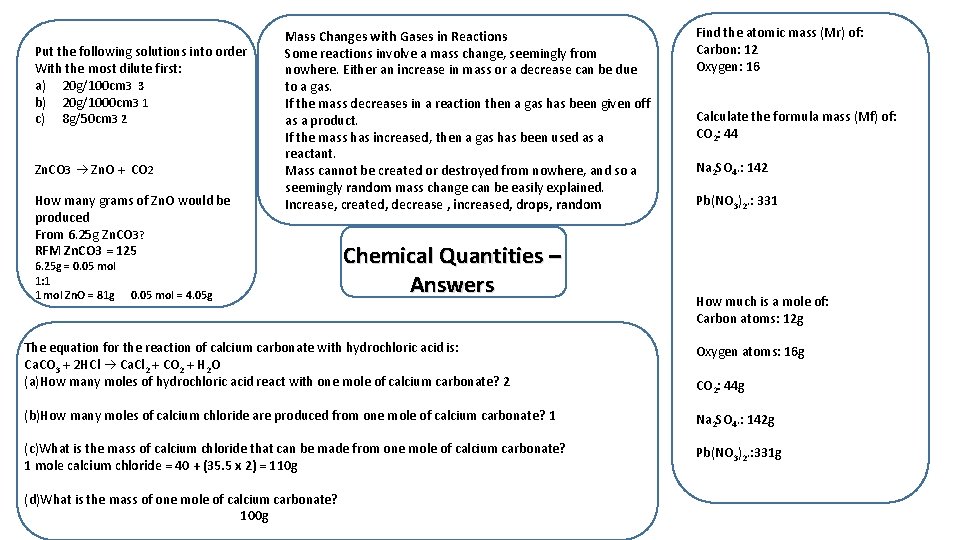
A group of students are heating magnesium In a crucible. At the start of the experiment The crucible and magnesium weigh 10 g. After heating for a few minutes The students let their crucible cool. The mass is now 11 g. Explain why: Magnesium has reacted with oxygen in the air to produce Magnesium oxide. Work out the mass of the missing reactant/product KOH + HBr —> KBr + H 2 O 13 g + 17 g —> 20 g + 10 g 2 H 2 SO 4 + 2 Cs. OH —> Cs 2 SO 4 + 2 H 2 O 17 g + 24 g —> 32 g + 19 g Balance the following equations: 2 H 2 + O 2 2 H 2 O 3 H 2 + N 2 2 NH 3 4 Cr + 3 O 2 2 Cr 2 O 3 Chemical Quantities. Answers 2 Na. Cl. O 3 2 Na. Cl + 3 O 2 Relative Atomic Mass (RAM, Ar) An atom’s mass number is the sum of its protons and neutrons. Carbon has 6 neutrons, and 6 protons so we say it has a mass of 12. Some elements have isotopes. These are the same atom, but with different numbers of neutrons. The Carbon-12 Standard The relative atomic mass of an element is the average mass of an atom of that element compared to the mass of an atom of carbon 12. Relative Formula Mass (RFM, Mr) The RFM of a compound is the sum of all the RAMs of the atoms in that compound. C 5 H 10 + 5 O 2 —> 5 CO + 5 H 2 O 50 g + 16 g —> 26 g + 40 g When magnesium is heated in air it gains mass. If 2. 4 g of magnesium makes 4 g of magnesium oxide, how much oxygen was added from the air? 1. 6 g A reaction is shown to lose mass during an experiment. Explain why. A gas is given off

Put the following solutions into order With the most dilute first: a) 20 g/100 cm 3 3 b) 20 g/1000 cm 3 1 c) 8 g/50 cm 3 2 Zn. CO 3 Zn. O + CO 2 How many grams of Zn. O would be produced From 6. 25 g Zn. CO 3? RFM Zn. CO 3 = 125 6. 25 g = 0. 05 mol 1: 1 1 mol Zn. O = 81 g Mass Changes with Gases in Reactions Some reactions involve a mass change, seemingly from nowhere. Either an increase in mass or a decrease can be due to a gas. If the mass decreases in a reaction then a gas has been given off as a product. If the mass has increased, then a gas has been used as a reactant. Mass cannot be created or destroyed from nowhere, and so a seemingly random mass change can be easily explained. Increase, created, decrease , increased, drops, random 0. 05 mol = 4. 05 g Chemical Quantities – Answers Find the atomic mass (Mr) of: Carbon: 12 Oxygen: 16 Calculate the formula mass (Mf) of: CO 2: 44 Na 2 SO 4. : 142 Pb(NO 3)2. : 331 How much is a mole of: Carbon atoms: 12 g The equation for the reaction of calcium carbonate with hydrochloric acid is: Ca. CO 3 + 2 HCl Ca. Cl 2 + CO 2 + H 2 O (a)How many moles of hydrochloric acid react with one mole of calcium carbonate? 2 Oxygen atoms: 16 g (b)How many moles of calcium chloride are produced from one mole of calcium carbonate? 1 Na 2 SO 4. : 142 g (c)What is the mass of calcium chloride that can be made from one mole of calcium carbonate? 1 mole calcium chloride = 40 + (35. 5 x 2) = 110 g Pb(NO 3)2. : 331 g (d)What is the mass of one mole of calcium carbonate? 100 g CO 2: 44 g