A Further Look at the Current Equivalence Test







- Slides: 26

A Further Look at the Current Equivalence Test for Analytical Similarity Assessment Neal Thomas, Ph. D, Statistics Research and Consulting Center, Pfizer Aili Cheng, Ph. D, Pharm Sci and PGS Statistics, Pfizer THE 39 th ANNUAL MIDWEST BIOPHARMACEUTICAL STATISTICS WORKSHOP MAY 16 – 18, 2016

What Are Biosimilars? A biosimilar is a new biologic product that is highly similar to an already approved product (the reference product) in terms of structure, function, and biological activity. “A biosimilar is a biopharmaceutical that contains a version of the active substance of an already authorized biopharmaceutical” “Similarity to the reference medicinal product in terms of quality characteristics, biological activity, safety, and efficacy needs to be established” 1 “Biosimilarity means the biologic product is highly similar to the reference product notwithstanding minor differences in clinically inactive components” “There are no clinically meaningful differences between the biologic product and the reference product in terms of the safety, purity and potency of the product” 2 1

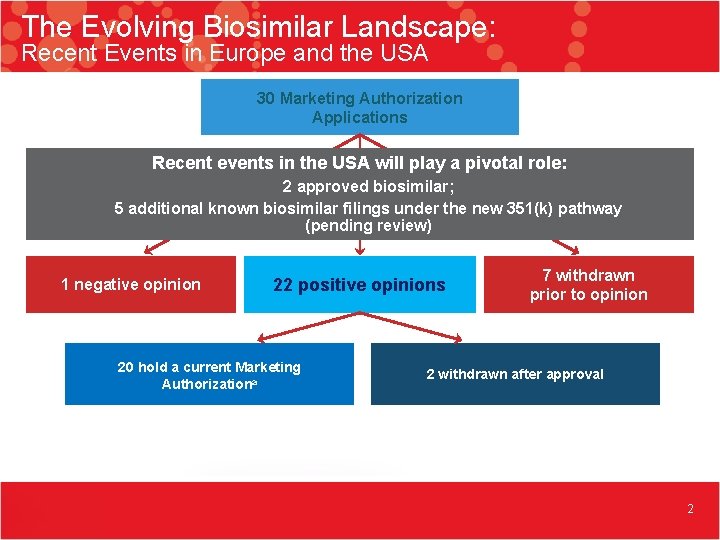
The Evolving Biosimilar Landscape: Recent Events in Europe and the USA 30 Marketing Authorization Applications Recent events in the USA will play a pivotal role: 2 approved biosimilar; 5 additional known biosimilar filings under the new 351(k) pathway (pending review) 1 negative opinion 22 positive opinions 20 hold a current Marketing Authorizationa 7 withdrawn prior to opinion 2 withdrawn after approval 2

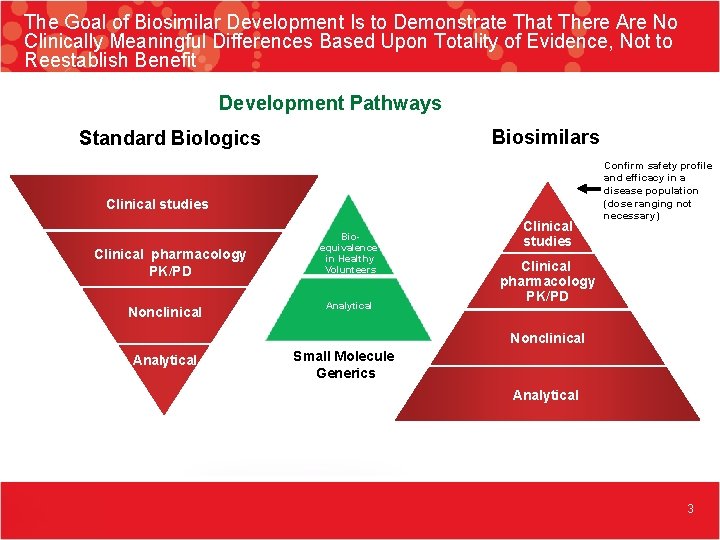
The Goal of Biosimilar Development Is to Demonstrate That There Are No Clinically Meaningful Differences Based Upon Totality of Evidence, Not to Reestablish Benefit Development Pathways Biosimilars Standard Biologics Clinical studies Clinical pharmacology PK/PD Nonclinical Bioequivalence in Healthy Volunteers Analytical Clinical studies Confirm safety profile and efficacy in a disease population (dose ranging not necessary) Clinical pharmacology PK/PD Nonclinical Analytical Small Molecule Generics Analytical 3

Outline • Introduction to FDA’s Tiered Analytical Assessment Approach • Assessment of FDA’s Recommended Equivalence Test – Asymptotic Derivation of the Test Statistic – The Impact of Sample Size and Margin Setting on Type I Error and Power – Sample Size Adjustment for Imbalanced Case • Summary 4

FDA Tiered Approach – Step 1: Criticality Assessment • Assign the quality attributes (QAs) to one of the three tiers mainly based on criticality risk ranking • Criticality risk ranking is obtained by ranking the QAs based on the potential impact on activity, PK/PD, safety, and immunogenicity 5

FDA Tiered Approach – Step 2: Assessment of Attributes: Tier 1: Equivalence test – Recommended for quality attributes with the highest risk ranking – Expected to be few attributes – Includes assays that evaluate clinically relevant mechanism(s) of action Tier 2: Quality range – – For QAs with lower risk ranking mean +/- X σ Based on the reference lots X should be justified Tier 3: Raw data /graphical comparisons – For QAs with lowest risk ranking 6

Some Special Requirements Recommended For Biosimilars • *Reference product: single biological licensed product developed by innovators 7

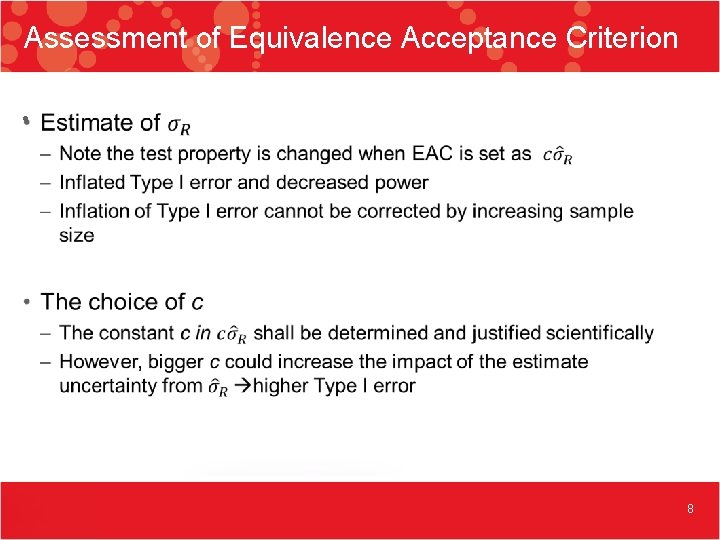
Assessment of Equivalence Acceptance Criterion • 8

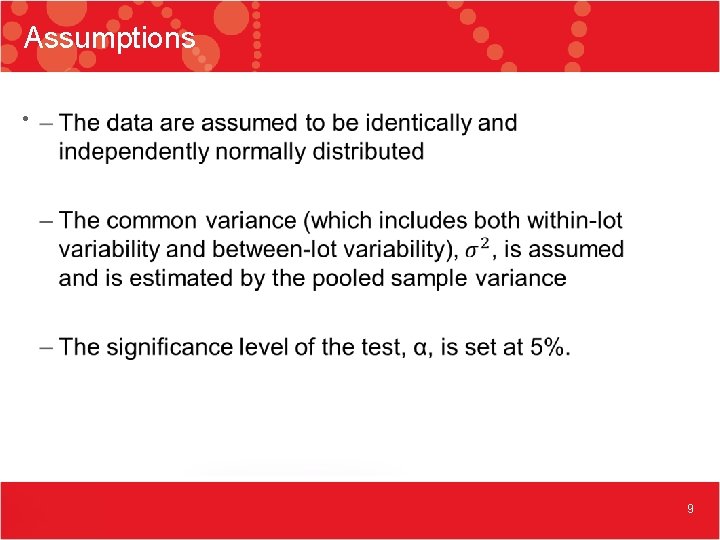
Assumptions • 9

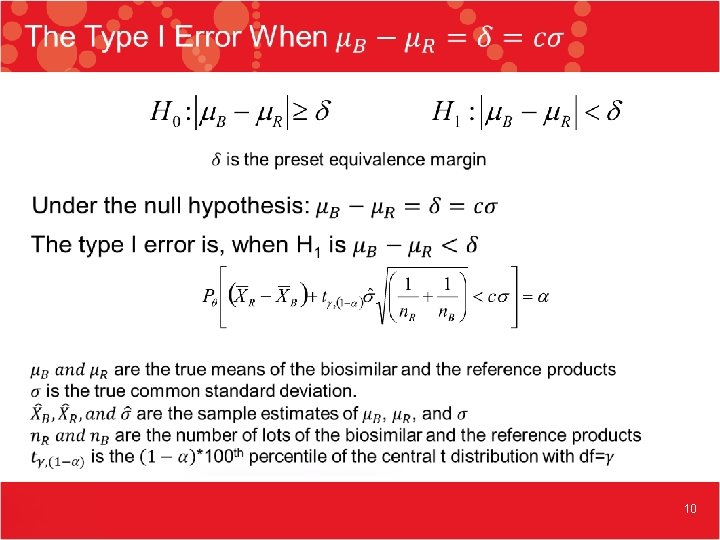
10

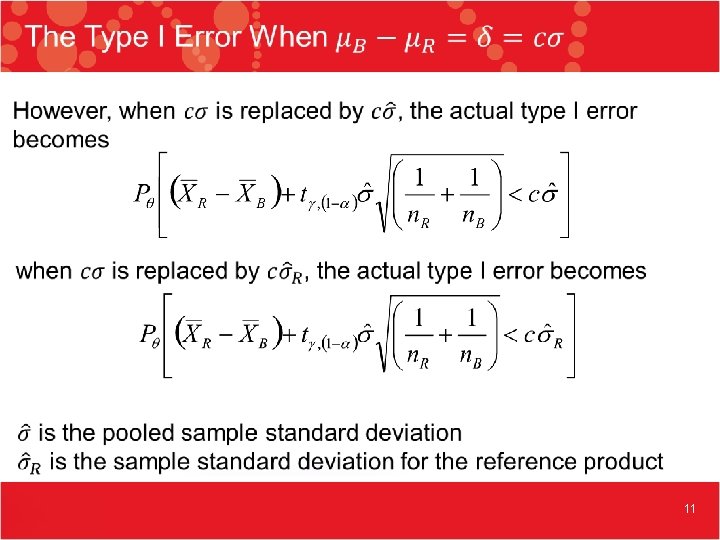
11

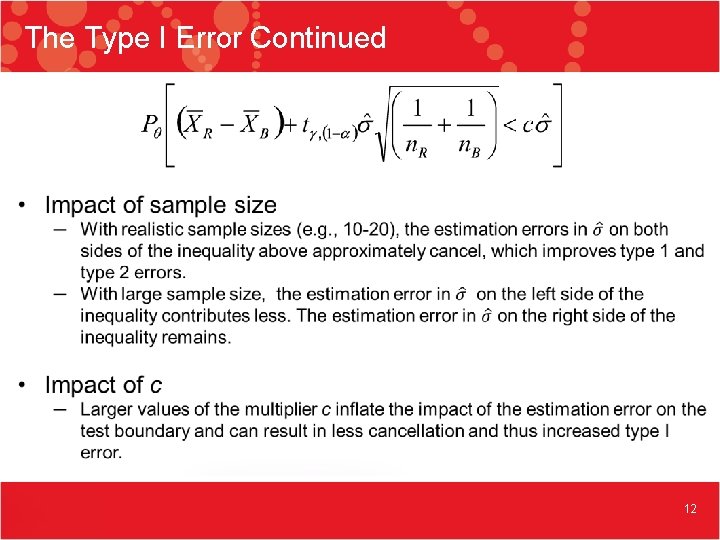
The Type I Error Continued 12

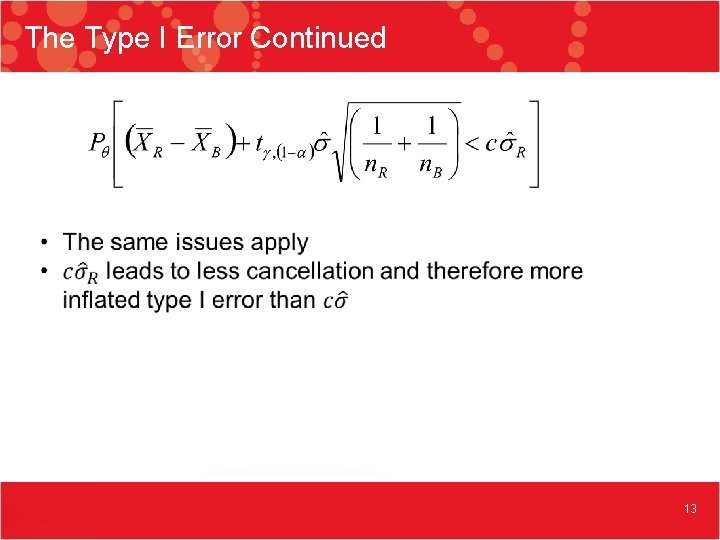
The Type I Error Continued 13

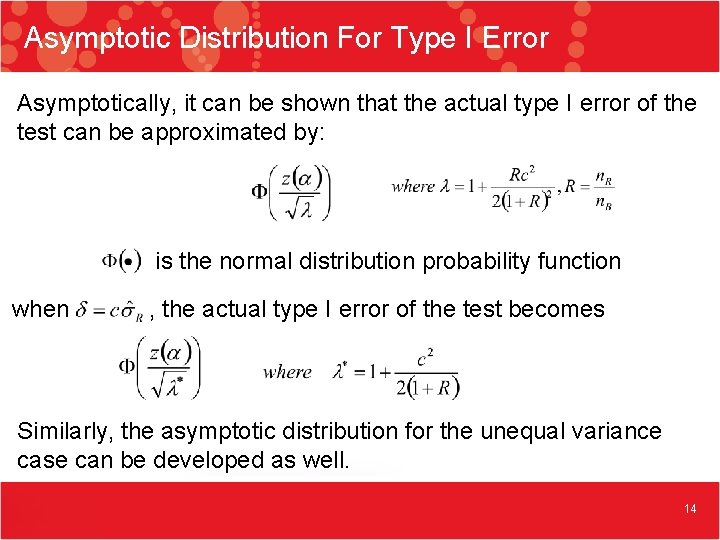
Asymptotic Distribution For Type I Error Asymptotically, it can be shown that the actual type I error of the test can be approximated by: is the normal distribution probability function when , the actual type I error of the test becomes Similarly, the asymptotic distribution for the unequal variance case can be developed as well. 14

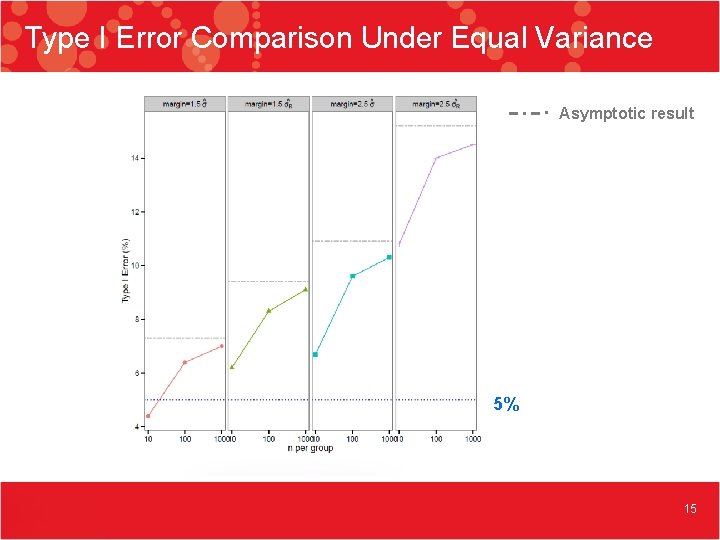
Type I Error Comparison Under Equal Variance Asymptotic result 5% 15

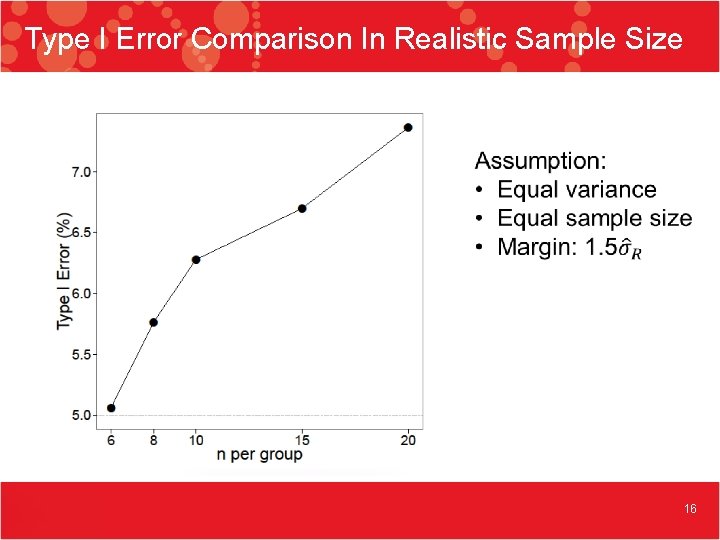
Type I Error Comparison In Realistic Sample Size 16

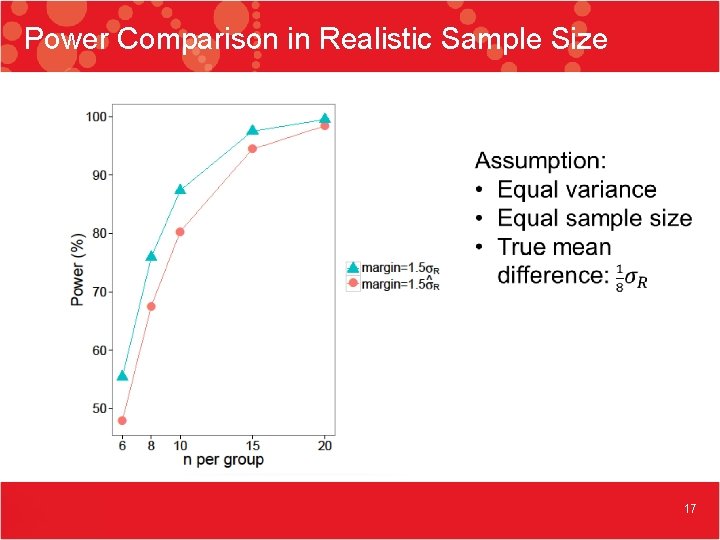
Power Comparison in Realistic Sample Size 17

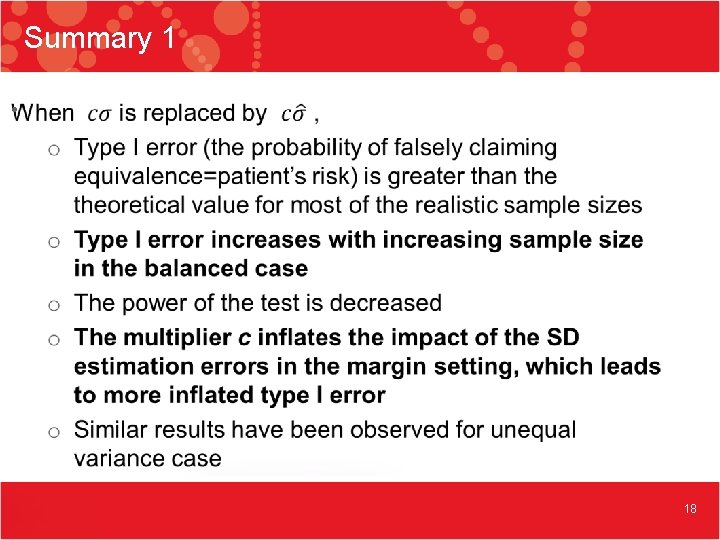
Summary 1 • 18

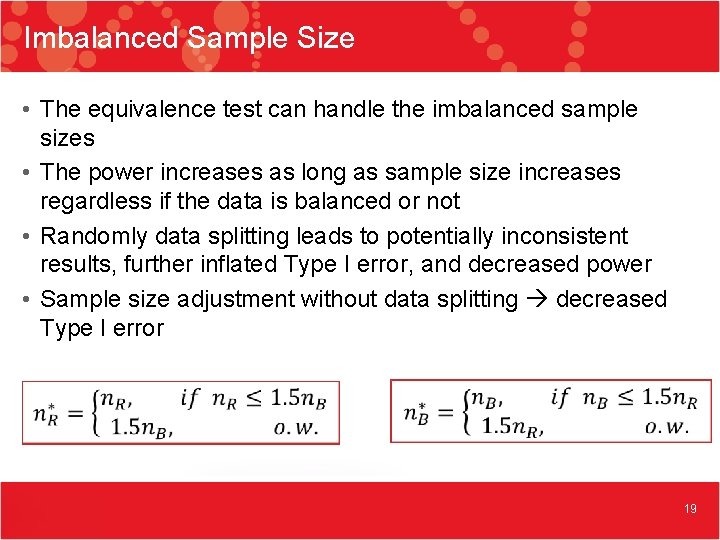
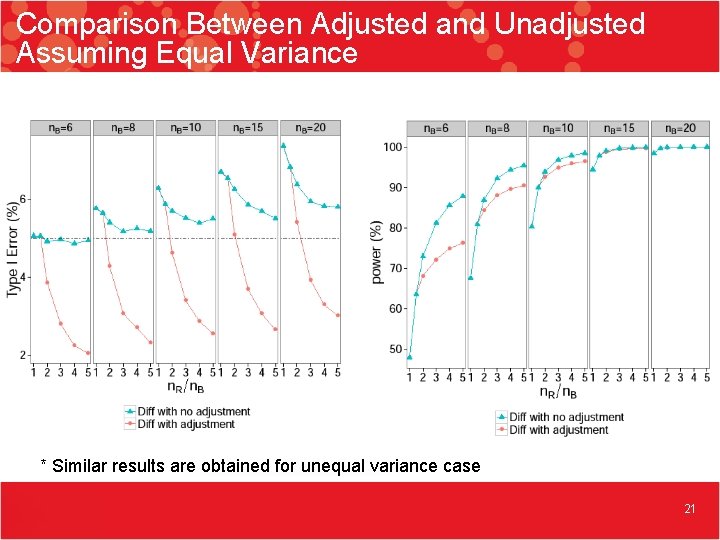
Imbalanced Sample Size • The equivalence test can handle the imbalanced sample sizes • The power increases as long as sample size increases regardless if the data is balanced or not • Randomly data splitting leads to potentially inconsistent results, further inflated Type I error, and decreased power • Sample size adjustment without data splitting decreased Type I error 19

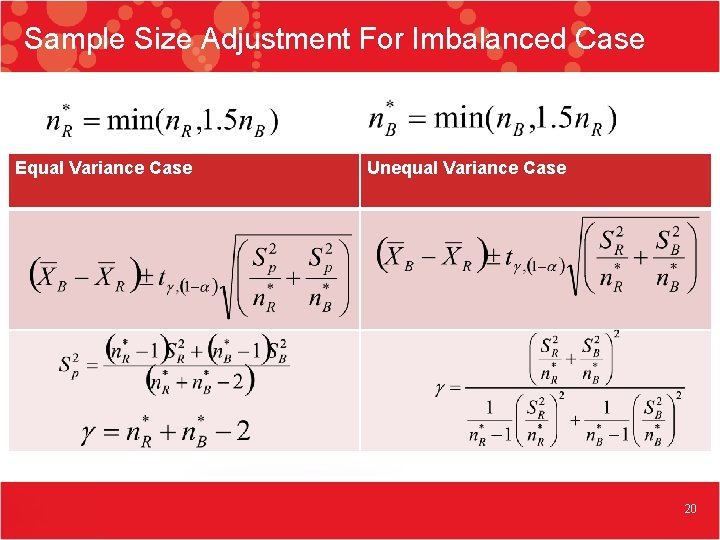
Sample Size Adjustment For Imbalanced Case Equal Variance Case Unequal Variance Case 20

Comparison Between Adjusted and Unadjusted Assuming Equal Variance * Similar results are obtained for unequal variance case 21

Summary 2 • The current equivalence test for Tier 1 quality attributes does not have proper statistical properties especially in Type I error, which cannot be corrected by increasing sample size • In the imbalanced case, the sample size adjustment tends to over-correct the Type I error issue especially for smaller sample size 22

Statistical Reference • Chow SC (2014) On Assessment of Analytical Similarity in Biosimilar Studies. Drug Des 3: e 124. doi: 10. 4172/2169 -0138. 1000 e 124 • Tsong, Y. , Shen, M. and Dong, X. (2015). Development of statistical approaches for analytical biosimilarity evaluation. 2015 DIA/FDA Statistical Forum, April, 2015, Rockville, MD. • Tsong, Y. , Shen, M. and Dong, X. (2015). Development of Statistical Approaches for Analytical Biosimilarity Evaluation, June 2015 ISBSDIA Joint Symposium on Biopharmaceutical Statistics, Beijing, China • Tsong, Y. , Shen, M. and Dong, X. (2015). Equivalence margin determination for analytical biosimilarity assessment. IABS Workshop at USP Headquarters, Sept, 2015, Rockville, MD. 23

Other Reference • • • • European Medicines Agency. Guideline on Similar Biological Medicinal Products Containing Biotechnology-derived Proteins as Active Substance: Nonclinical and Clinical Issues. 2014. Accessed August 2015. US Food and Drug Administration. Guidance for Industry: Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. Rockville, MD: FDA; 2015. Schneider CK, et al. Nat Biotechnol. 2012; 30: 1179 -1185. Mc. Camish M. Presented at EMA Workshop on Biosimilars; London; October 2013. Berghout A. Biologicals. 2011; 39: 293 -296. US Food and Drug Adminstration. Abbreviated New Drug Applications (ANDA): Generics. http: //www. fda. gov/Drugs/Development. Approval. Process/How. Drugsare. Developedand. Approved/Approval. Applications/Abbreviated. New. Drug. Application. A NDAGenerics/. Accessed January 3, 2016. European Medicines Agency Website. European Public Assessment Reports. http: //www. ema. europa. eu/ema/index. jsp? curl=pages%2 Fmedicines%2 Flanding%2 Fepar_search. jsp&mid=WC 0 b 01 ac 058001 d 124&search. Tab=search By. Auth. Type&already. Loaded=true&is. New. Query=true&status= Authorised&status=Withdrawn&status=Suspended&status=Refused&keyword=Enter+keywords&search. Type=name&taxonomy. Path=&tree. Number=&s earch. Generic. Type=biosimilars&generics. Keyword. Search=Submit. Accessed January 4, 2016. FDA accepts Sandoz application for biosimilar filgrastim [press release]. July 24, 2014. http: //www. sandoz. com/media_center/press_releases_news/global_news/2014_07_24_FDA_accepts_Sandoz_application_for_biosimilar_ filgrastim. shtml. Accessed January 4 , 2016. Celltrion files for US FDA approval of Remsima [press release]. August 11, 2014. http: //www. celltrion. com/en/company/ notice_view. asp? idx=456&code=ennews&int. Now. Page=1&menu_num=&align_year=all. Accessed January 4, 2016. Apotex announces FDA has accepted for filing its biosimilar application for pegfilgrastim [press release]. December 17, 2014. http: //www. apotex. com/global/about/press/ 20141217. asp. Accessed January 4, 2016. FDA to review insulin biosimilar. Ga. BI Online. January 31, 2014. http: //www. gabionline. net/Biosimilars/News/FDA-to-review-insulin-biosimilar. Accessed January 4, 2016. Hospira submits new biologics license application to U. S. FDA for proposed epoetin alfa biosimilar [press release]. Lake Forest, IL: Hospira, Inc. ; January 12, 2015. http: //www. prnewswire. com/news-releases/hospira-submits-new-biologics-license-application-to-us-fda-for-proposed-epoetin-alfabiosimilar-300018991. html. Accessed January 4, 2016. Amgen’s first biosimilar BLA for ABP 501 submitted to US FDA. http: //www. amgen. com/media/news-releases/2015/11/amgens-first-biosimilar-biologics -license-application-for-abp-501 -submitted-to-us-food-and-drug-administration/Accessed January 5, 2016. 24

Acknowledgement • • Kim Vukovinsky Ira Jacobs Andrew Rugaiganisa Brad Evans Jennifer Chen Leah Isakov Chee-Keng Ng 25