A Comparative CFD Assessment Study of Cryogenic Hydrogen







- Slides: 17

A Comparative CFD Assessment Study of Cryogenic Hydrogen and Liquid Natural Gas Dispersion S. G. Giannissi 1 and A. G. Venetsanos 1 1 Environmental Research Laboratory, National Centre for Scientific Research Demokritos, Aghia Paraskevi, Athens, 15341, Greece, sgiannissi@ipta. demokritos. gr, venets@ipta. demokritos. gr ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany

Introduction § Hydrogen is a key-element of potential energy solution for 21 st century. § Natural gas is widely used since 2 nd world war. § Storage practice under cryogenic conditions (liquefaction or cryo -compressed technology) § Need in studying the physical phenomena associated with cryogenic releases § Due to extreme low temperature atmospheric humidity, nitrogen and oxygen are condensed and solidified 13 Sep. 2017 ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany 2/16

Introduction § There are no experiments to study the humidity and air component’s phase change effect. § Few CFD studies have examined these effects in LH 2 spills, Ichard et al. 2012, Giannissi et al. 2014, and LNG spills, Giannissi et al. 2015, Zhang et al. 2015. § However, no study on the level of influence depending on the relative humidity and the release conditions has not been conducted. § In cryo-compressed hydrogen (CCH 2) the effect has not been examined yet. 13 Sep. 2017 ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany 3/16

Scope of study § Study the humidity and air’s component phase change effect on mixture dispersion under cryogenic release conditions (both liquid hydrogen (LH 2) and cryo-compressed hydrogen (CCH 2) ). § Study the humidity effect on liquefied natural gas (LNG) dispersion § Identify any differences among the behavior of LH 2, CCH 2 and LNG under the influence of these phenomena. 13 Sep. 2017 ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany 4/16

Problem setup § To conduct the study we performed several simulations with the following characteristics: Ø Cryogenic fluid (H 2 or CH 4) release horizontally 1 m above the ground. Ø Stagnant environment. Ø Sensitivity tests on relative humidity levels. Ø Sensitivity tests on release conditions. 13 Sep. 2017 ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany 5/16

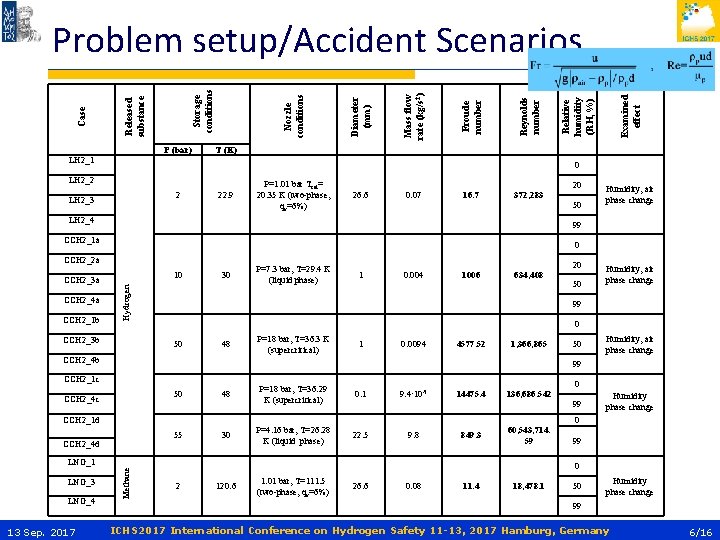
P (bar) 0 LH 2_2 2 LH 2_3 22. 9 P=1. 01 bar Tsat= 20. 35 K (two-phase, qv=6%) 26. 6 0. 07 16. 7 372, 283 LH 2_4 30 P=7. 3 bar, T=29. 4 K (liquid phase) 1 0. 004 1006 634, 408 50 48 50 Humidity, air phase change P=18 bar, T=36. 3 K (supercritical) 1 0. 0094 4577. 52 1, 366, 865 50 Humidity, air phase change 99 CCH 2_1 c 50 CCH 2_4 c 48 P=18 bar, T=36. 29 K (supercritical) 30 P=4. 16 bar, T=26. 28 K (liquid phase) 120. 6 1. 01 bar, T=111. 5 (two-phase, qv=6%) 0 0. 1 9. 4∙ 10 -5 14475. 4 136, 686. 542 849. 3 60, 543, 714. 59 CCH 2_1 d 55 CCH 2_4 d 22. 5 9. 8 99 Humidity phase change 0 99 0 Methane LNG_1 13 Sep. 2017 20 0 CCH 2_4 b LNG_4 Humidity, air phase change 99 Hydrogen 10 CCH 2_3 b LNG_3 50 0 CCH 2_2 a CCH 2_1 b 20 99 CCH 2_1 a CCH 2_4 a Examined effect T (K) LH 2_1 CCH 2_3 a Relative humidity (RH, %) Reynolds number Froude number Mass flow rate (kg/s 2) Diameter (mm) Nozzle conditions Storage conditions Released substance Case Problem setup/Accident Scenarios 2 26. 6 0. 08 11. 4 18, 478. 1 50 Humidity phase change 99 ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany 6/16

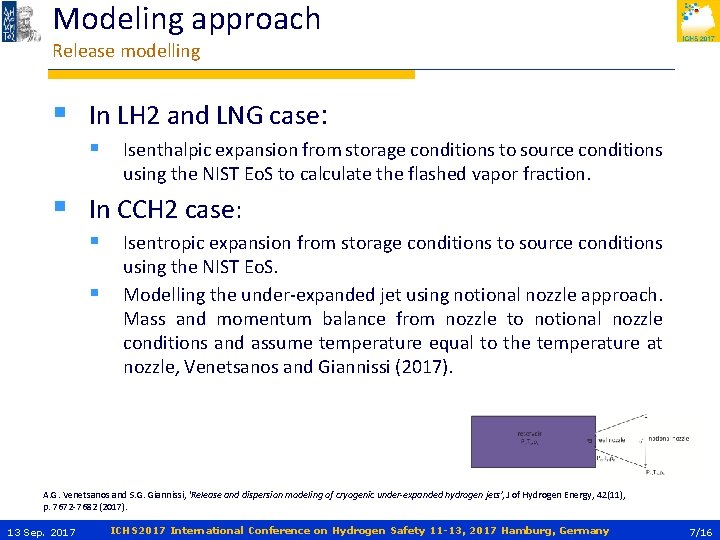
Modeling approach Release modelling § In LH 2 and LNG case: § Isenthalpic expansion from storage conditions to source conditions using the NIST Eo. S to calculate the flashed vapor fraction. § In CCH 2 case: § Isentropic expansion from storage conditions to source conditions § using the NIST Eo. S. Modelling the under-expanded jet using notional nozzle approach. Mass and momentum balance from nozzle to notional nozzle conditions and assume temperature equal to the temperature at nozzle, Venetsanos and Giannissi (2017). A. G. Venetsanos and S. G. Giannissi, ‘Release and dispersion modeling of cryogenic under-expanded hydrogen jets’, J of Hydrogen Energy, 42(11), p. 7672 -7682 (2017). 13 Sep. 2017 ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany 7/16

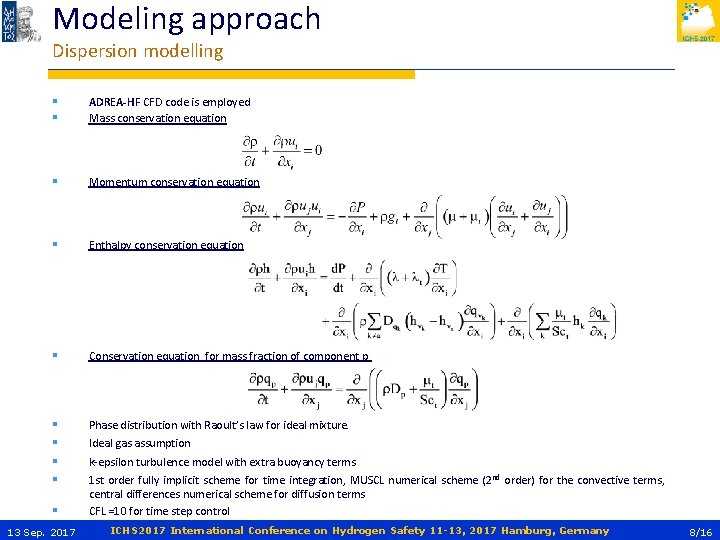
Modeling approach Dispersion modelling § § ADREA-HF CFD code is employed Mass conservation equation § Momentum conservation equation § Enthalpy conservation equation § Conservation equation for mass fraction of component p § § Phase distribution with Raoult’s law for ideal mixture. § 13 Sep. 2017 Ideal gas assumption k-epsilon turbulence model with extra buoyancy terms 1 st order fully implicit scheme for time integration, MUSCL numerical scheme (2 nd order) for the convective terms, central differences numerical scheme for diffusion terms CFL =10 for time step control ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany 8/16

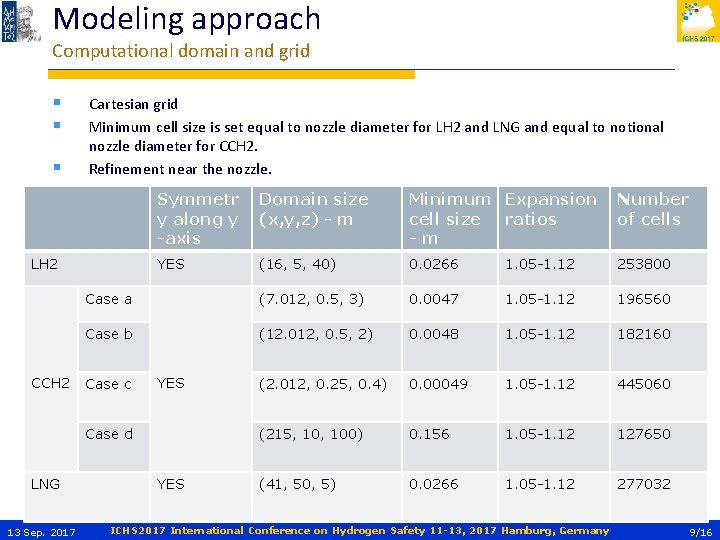
Modeling approach Computational domain and grid § § § Cartesian grid Minimum cell size is set equal to nozzle diameter for LH 2 and LNG and equal to notional nozzle diameter for CCH 2. Refinement near the nozzle. Symmetr y along y -axis Domain size (x, y, z) - m Minimum Expansion cell size ratios -m Number of cells YES (16, 5, 40) 0. 0266 1. 05 -1. 12 253800 Case a (7. 012, 0. 5, 3) 0. 0047 1. 05 -1. 12 196560 Case b (12. 012, 0. 5, 2) 0. 0048 1. 05 -1. 12 182160 (2. 012, 0. 25, 0. 4) 0. 00049 1. 05 -1. 12 445060 (215, 100) 0. 156 1. 05 -1. 12 127650 (41, 50, 5) 0. 0266 1. 05 -1. 12 277032 LH 2 CCH 2 Case c YES Case d LNG 13 Sep. 2017 YES ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany 9/16

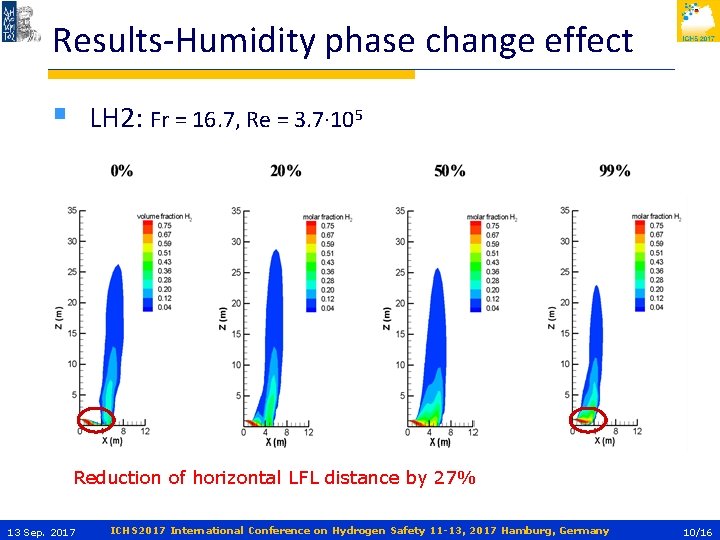
Results-Humidity phase change effect § LH 2: Fr = 16. 7, Re = 3. 7∙ 105 Reduction of horizontal LFL distance by 27% 13 Sep. 2017 ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany 10/16

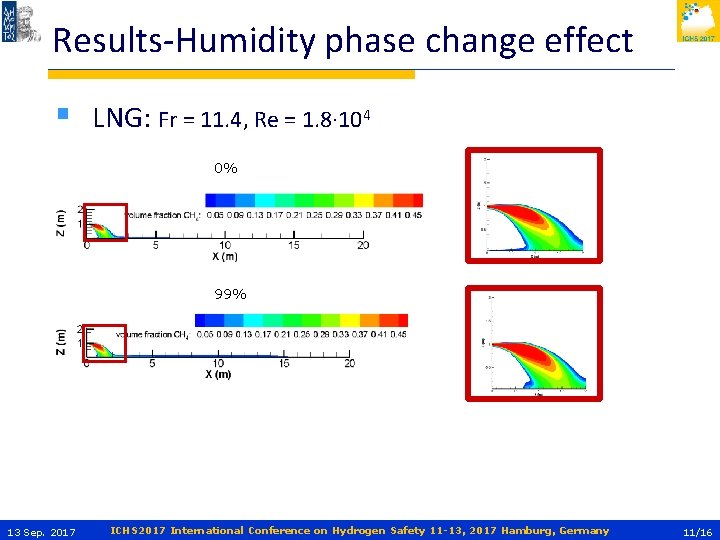
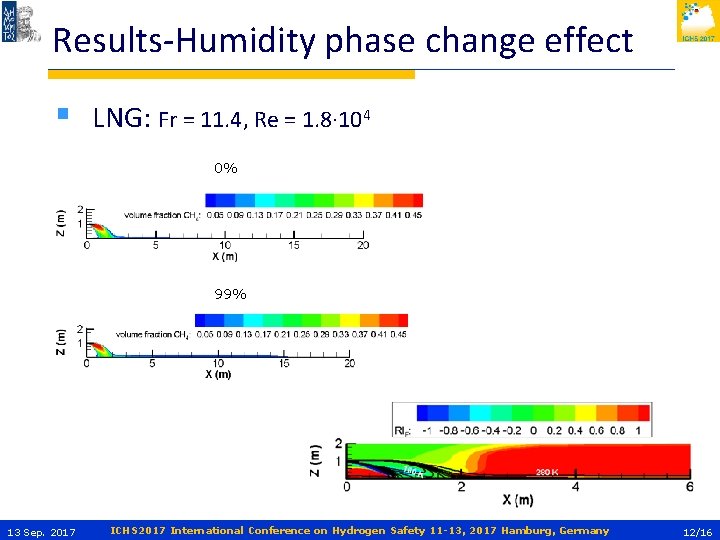
Results-Humidity phase change effect § LNG: Fr = 11. 4, Re = 1. 8∙ 104 0% 99% 13 Sep. 2017 ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany 11/16

Results-Humidity phase change effect § LNG: Fr = 11. 4, Re = 1. 8∙ 104 0% 99% 13 Sep. 2017 ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany 12/16

Results-Humidity phase change effect § CCH 2 Fr = 1006 Re = 6. 3∙ 105 Reduction of horizontal LFL distance by 25% Fr = 4577 Re = 1. 4 ∙ 106 Fr = 14475. 4 Re = 1. 4 ∙ 105 Fr = 849 Re = 6. 0 ∙ 107 13 Sep. 2017 Reduction of horizontal LFL distance by 30% ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany 13/16

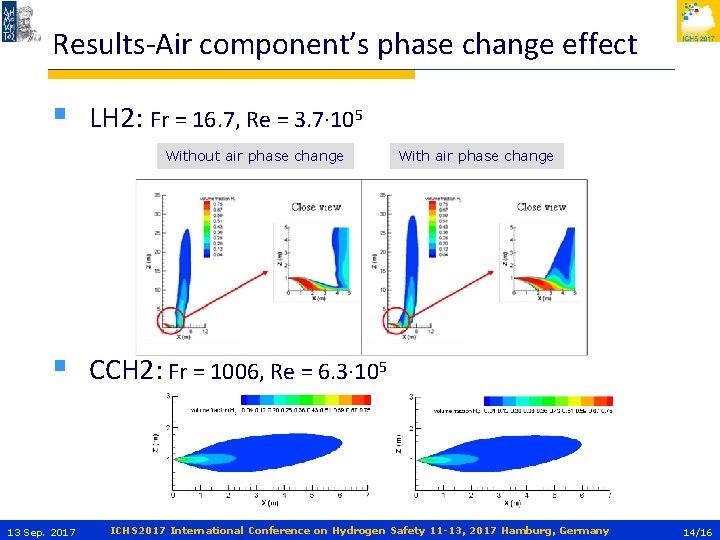
Results-Air component’s phase change effect § LH 2: Fr = 16. 7, Re = 3. 7∙ 105 Without air phase change With air phase change § CCH 2: Fr = 1006, Re = 6. 3∙ 105 13 Sep. 2017 ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany 14/16

Conclusions and Future work (1/2) § A CFD study of the humidity effect on liquid hydrogen § § § 13 Sep. 2017 (LH 2), cryo-compressed hydrogen (CCH 2) and liquid natural gas (LNG) dispersion in stagnant environment is carried out. In LH 2 and CCH 2 releases the effect of air components’ phase change is also examined. In hydrogen the condensation/freezing of humidity makes the cloud more buoyant hence the longitudinal Lower Flammability Limit (LFL) distance is significantly reduced (approximately 27% for ambient temperature equal to 288. 15 and relative humidity 99%). Higher specific humidity levels results in more enhanced buoyancy effect. ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany 15/16

Conclusions and Future work (2/2) § In order the humidity effect to be significant we should have low § § § 13 Sep. 2017 Fr number at nozzle (<1000). That is achieved if storage conditions correspond to liquid state or supercritical state in the side of the saturated liquid line. In LNG the buoyancy effect in presence of humidity is not pronounced. However, the LFL horizontal distance seems to be increased in presence of humidity. More study for the reasons of this behavior should be conducted. The air component phase change is perceptible in LH 2 spills, while it is negligible in CCH 2 releases due to the high jet momentum. In the future, a critical value of Fr number for the humidity effect to be significant could be investigated. ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany 16/16

Thank you very much for your attention The first author would like to acknowledge the “IKY FELLOWSHIPS OF EXCELLANCE FOR POSTGRADUATE STUDIES IN GREECE-SIEMENS PROGRAM” for the financial support. The authors would also like to thank the SUSANA project, which has received funding from the European Union's Seventh Framework Programme (FP 7/2007 -2013) for the Fuel Cells and Hydrogen Joint Technology Initiative under grant agreement n° FCH-JU-325386. ICHS 2017 International Conference on Hydrogen Safety 11 -13, 2017 Hamburg, Germany