A Comparative Analysis of Prevagen Supplementation and Current












- Slides: 24

A Comparative Analysis of PrevagenⓇ Supplementation and Current Nootropic Agents Collin Belk, Kirsten Carstarphen, Jace Engstler, Kelly Ferris

• “Prevagen is a dietary supplement that has been clinically shown to help with mild memory problems associated with aging. Prevagen contains apoaequorin, which is safe and uniquely supports brain function” • Manufactured and marketed by Quincy Bioscience starting in 2007 • Improves memory and supports: • Healthy brain function • Sharper mind • Clearer thinking Prevagen Story – Background/Purpose • Available as oral capsules and chewables • Regular strength - 10 mg • Extra strength - 20 mg

Study Objectives • • Evaluate the claims made about Prevagen by Quincy Bioscience, and determine through research and literature evaluations if these claims are scientifically supported. Compare FDA approved drug targets for nootropic agents and how they relate to the mechanism proposed by the makers of Prevagen. Methods • • A literature review and critiques of Prevagen A comparison of nootropic agents, their mechanism of action, pharmacological basis and how it relates to Prevagen’s proposed mechanism and biochemical properties.

Results (keep as a title slide? )

What would make a good nootropic agent? • Molecular weight < 500 Da • Neutral or basic molecule with p. Ka between 7. 5 -10. 5 • Log. P < 5 • Polar surface area < 60 -70 Å 2 • Number of hydrogen bonds in molecule: < 8 • The sum of nitrogen and oxygen atoms in molecule: ≤ 5

History Apoaequorin History and Rationale • Natural, bio-luminescent calcium-binding protein found in jellyfish • Historically has been used as a calcium-sensor for research Calcium • Secondary messenger with many important physiological functions • Ex: Synaptic and neuronal plasticity, enzyme activation, regulation of gene expression • Endogenous calcium-binding proteins help regulated Ca 2+ levels • As we age, Ca 2+ homeostasis is not maintained as effectively • Elevated intracellular levels • Decreased levels of calcium-binding proteins

Digestion • • Apoaequorin – Biological Plausibility • Orally ingested proteins are highly digestible No evidence to support apoaequorin surviving digestion Simulated gastric environment - 90% digested in 30 seconds Bioavailability • • • Sharp decrease as molecular weights eclipse 500 -700 Da Apoaequorin = 22. 3 k. Da with 196 amino acid residues No evidence to support absorption Blood-Brain Barrier • • No evidence of transporters or receptors Cut-off for transmembrane diffusion is often propos

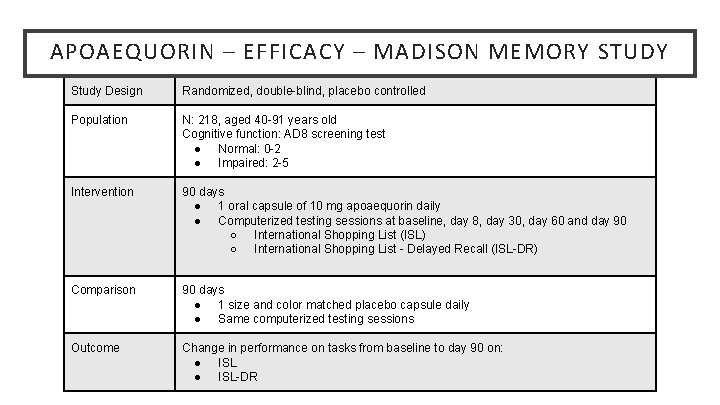
APOAEQUORIN – EFFICACY – MADISON MEMORY STUDY Study Design Randomized, double-blind, placebo controlled Population N: 218, aged 40 -91 years old Cognitive function: AD 8 screening test ● Normal: 0 -2 ● Impaired: 2 -5 Intervention 90 days ● 1 oral capsule of 10 mg apoaequorin daily ● Computerized testing sessions at baseline, day 8, day 30, day 60 and day 90 ○ International Shopping List (ISL) ○ International Shopping List - Delayed Recall (ISL-DR) Comparison 90 days ● 1 size and color matched placebo capsule daily ● Same computerized testing sessions Outcome Change in performance on tasks from baseline to day 90 on: ● ISL-DR

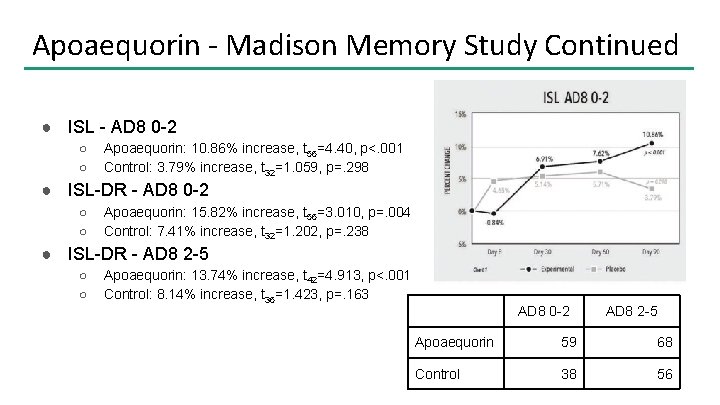
Apoaequorin - Madison Memory Study Continued ● ISL - AD 8 0 -2 ○ ○ Apoaequorin: 10. 86% increase, t 56=4. 40, p<. 001 Control: 3. 79% increase, t 32=1. 059, p=. 298 ● ISL-DR - AD 8 0 -2 ○ ○ Apoaequorin: 15. 82% increase, t 56=3. 010, p=. 004 Control: 7. 41% increase, t 32=1. 202, p=. 238 ● ISL-DR - AD 8 2 -5 ○ ○ Apoaequorin: 13. 74% increase, t 42=4. 913, p<. 001 Control: 8. 14% increase, t 36=1. 423, p=. 163 AD 8 0 -2 AD 8 2 -5 Apoaequorin 59 68 Control 38 56

APOAEQUORIN - MADISON MEMORY STUDY CONTINUED • Limitations o Subjective bias and conflict of interest § All authors are Quincy Bioscience employees § No mention of independent party assistance with randomization, blinding or statistics § Paid advertisement in journal o Did not directly compare apoaequorin to placebo to show it worked better § Only provided paired t test results o Paired t test degrees of freedom different that would be expected § Data from 51 subjects is missing o Did not provide evidence or rationale for apoaequorin’s mechanism in reaching therapeutic target

Amphetamine Salts - Methylphenidate • MOA: Block the reuptake of norepinephrine and dopamine into the presynaptic neuron, and increase their release into extraneuronal space • Place in therapy: Both agents FDA approved for the treatment of ADHD • Known as “smart pills” that are believed to enhance cognitive ability • New York Times, Wall Street Journal, Vogue, & CNN all have reported a trend toward growing use of stimulants by healthy people for the purpose of enhancing school or work performance

• Reviewed data on prescription stimulants as neuroenhancers from over forty laboratory Studies of subjects who were considered healthy, non-elderly adults • Studies varied in many ways • Type of tasks used Studies • Specific drug used • Dosage determination (weight dependant or dose dependant) • Sample size • Subject characteristics

Amphetamine Salts - Methylphenidate Studies 3 Types of Cognition 1) 2) 3) Learning a) Ex: memorizing a list of words for a designated amount of time b) Results: Some enhancement Working Memory a) Considered a temporary store of information that has a role in executive function i) Ex: series of items is presented to the subject for repetition, transcription, or recognition ii) Results: Some enhancement for lower performers Cognitive control a) Refers to the guidance of cognitive processes. Attention and working memory are thought to rely on cognitive control i) Ex: go/no-go task, the stop-signal task, and the Flanker test ii) Results: Some benefit but limited support for general population

Memantine MOA: Place in therapy: Impact on Cognitive Function: Studies to support

Donepezil Drug Class: Acetylcholinesterase inhibitor (ACh. EI) MOA: Inhibits the key enzyme, ACh. E, responsible for the breakdown of acetylcholine. Physiological basis for acetylcholine : • • • Plays many roles as an inhibitory or excitatory neurotransmitter depending on the receptor it binds to (e. g. , muscarinic, nicotinic) Acetylcholine plays an important role in the enhancement of alertness, sustaining attention and in learning/memory processes in the CNS. A lack of ACh in the basal forebrain is associated with cognitive dysfunction

Place in therapy: Donepezil Continued • Approved by FDA in 1996 for the treatment of Alzheimer Disease (mild, moderate or severe) Nootropic Properties: • Good bioavailability with decent BBB penetration • Small molecule (415. 96 k. DA) • Log. P < 5 (3. 6)

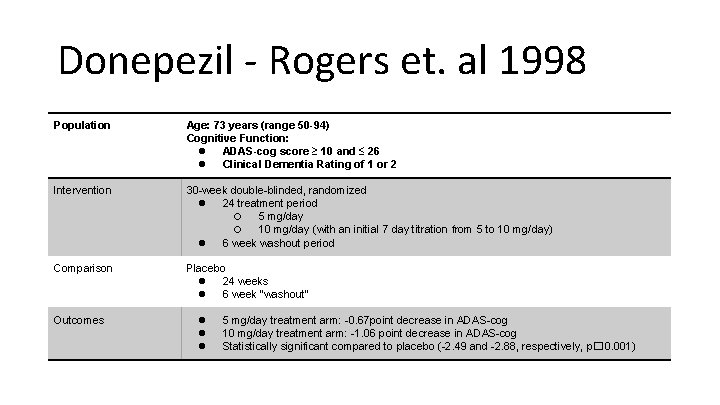
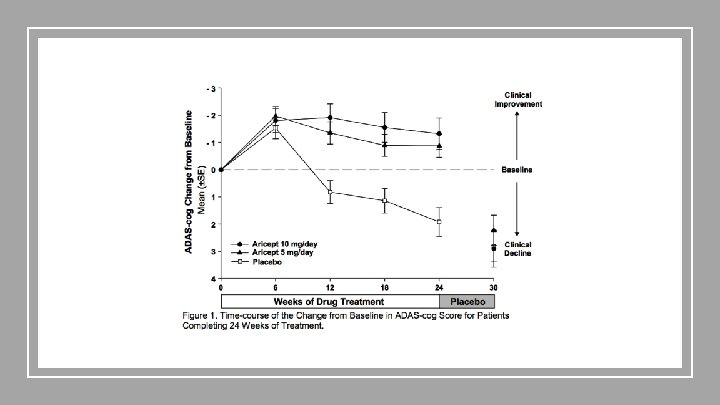
Donepezil - Rogers et. al 1998 Population Age: 73 years (range 50 -94) Cognitive Function: ● ADAS-cog score ≥ 10 and ≤ 26 ● Clinical Dementia Rating of 1 or 2 Intervention 30 -week double-blinded, randomized ● 24 treatment period ○ 5 mg/day ○ 10 mg/day (with an initial 7 day titration from 5 to 10 mg/day) ● 6 week washout period Comparison Placebo ● 24 weeks ● 6 week “washout” Outcomes ● ● ● 5 mg/day treatment arm: -0. 67 point decrease in ADAS-cog 10 mg/day treatment arm: -1. 06 point decrease in ADAS-cog Statistically significant compared to placebo (-2. 49 and -2. 88, respectively, p� 0. 001)


Donepezil in Healthy Adults Population Intervention Comparison Outcomes N= 24 young, healthy males, aged 19 -34 years, BMI between 20 -25, graduate/post-graduate students

Drug Class: Central Nervous System Stimulant Modafinil MOA: Exact mechanism unknown. Binds to the dopamine transporter, thereby inhibiting dopamine reuptake Place in therapy: FDA approved for the treatment of narcolepsy, obstructive sleep apnea, and shift work sleep disorder Nootropic Properties: Molecular Weight: 273 k. Da Log. P: 0. 6

Modafinil Studies Systematic review of cognitive neuroenhancement in healthy non-sleep-deprived subjects • Characteristics of included studies • 24 placebo-controlled studies • Study Design: parallel and cross-over • Sample sizes ranged from 10 to 64 participants • Modafinil dose ranged from 100 mg to 400 mg (over half of studies used 200 mg)

Modafinil Studies Continued Cognitive domains assessed 1) Attention a) Ex: Participants must detect letters/numbers and then determine whether they are immediately followed by another, related, letter or number. b) Results: The majority of studies showed no improvement in selective, sustained, or divided attention 2) Executive function a) Ex: Participants must respond rapidly to visually-presented “go” signals with a key stroke, and inhibit any response when a “stop” signal is suddenly presented b) Results: Some benefits in simple assessments, but significant effects in higher executive functions such as planning, decision making, and fluid intelligence 3) Learning and memory a) Ex: Participants are required to immediately repeat a list of digits in reverse order, presented visually or verbally b) Results: Some benefits were observed for short-term memory tasks, although an equal number of studies failed to observe any effect

Discussion Can we answer the question? - Does Prevagen compare to current nootropic agents? Limitations Prevagen is a supplement not regulated by the FDA for efficacy… Not all studies can be directly compared • Otherwise healthy individuals vs. those with cognitive dysfunction • Many ways to measure cognitive function - not all are standardized, not all are comparable to each other (i. e. , measure different aspects of cognitive function)

Exploration of other natural products used to enhance memory/cognition Future Directions Effects of commonly used cognitive enhancers such as caffeine and drugs of abuse. What are the current recommendations in the community? Community pharmacists’ input on Prevagen How do pharmacists in this practice setting handle this question? What pharmacies endorse the sale of Prevagen or other “memory enhancing” supplements