A combination of two or more substances that

A combination of two or more substances that are not chemically combined and can be separated by physical means.

A solution is a homogeneous mixture of two or more substances in a single phase. Thoroughly mixed Same Composition Throughout Same Properties Throughout

What makes up a solution? • Solute: the substance being dissolved • Solvent: the substance doing the dissolving Name the solute and solvent: solution solute(s) solvent Ocean water salt water Coca-cola Sugar, carbon dioxide water Humid air Water vapor air
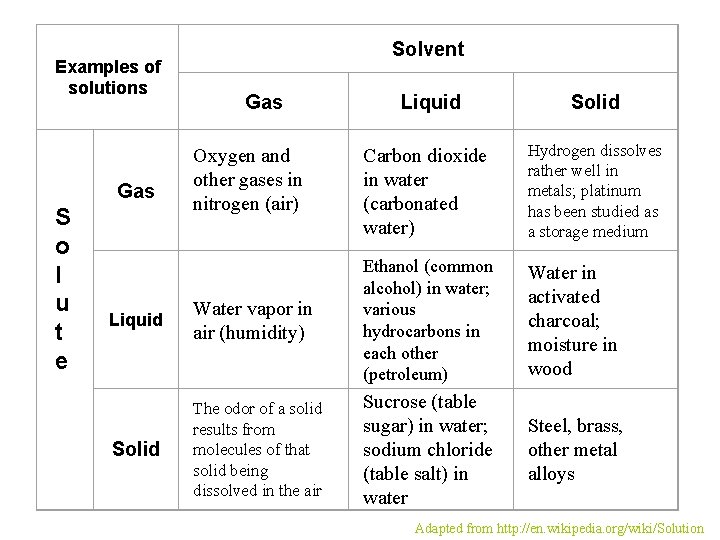
Examples of solutions Gas S o l u t e Solvent Gas Oxygen and other gases in nitrogen (air) Liquid Solid Carbon dioxide in water (carbonated water) Hydrogen dissolves rather well in metals; platinum has been studied as a storage medium Water in activated charcoal; moisture in wood Steel, brass, other metal alloys Liquid Water vapor in air (humidity) Ethanol (common alcohol) in water; various hydrocarbons in each other (petroleum) Solid The odor of a solid results from molecules of that solid being dissolved in the air Sucrose (table sugar) in water; sodium chloride (table salt) in water Adapted from http: //en. wikipedia. org/wiki/Solution
What types of solutions are there? • Aqueous solutions (aq): solutions in which water is the solvent. • Electrolytes: specific aqueous solutions in which the solute dissolves to form ions. • Tinctures: solutions in which alcohol is the solvent. • Alloys: solid solution of two or more metals. • Amalgams: specific alloys in which one of the metals is mercury.

If the particles in a solvent are so large that they settle out unless the mixture is constantly stirred or agitated, the mixture is called a suspension. Muddy Water

Particles that are intermediate in size between those in solutions and suspensions form mixtures known as colloidal dispersions, or colloids. paints, mud, gelatin, milk, mayonnaise, shaving cream, smoke, fog, butter

• A beam of light distinguishes a colloid from a solution. • The particles in a colloid will scatter light, making the beam visible. Na. Cl Solution Gelatin and Water
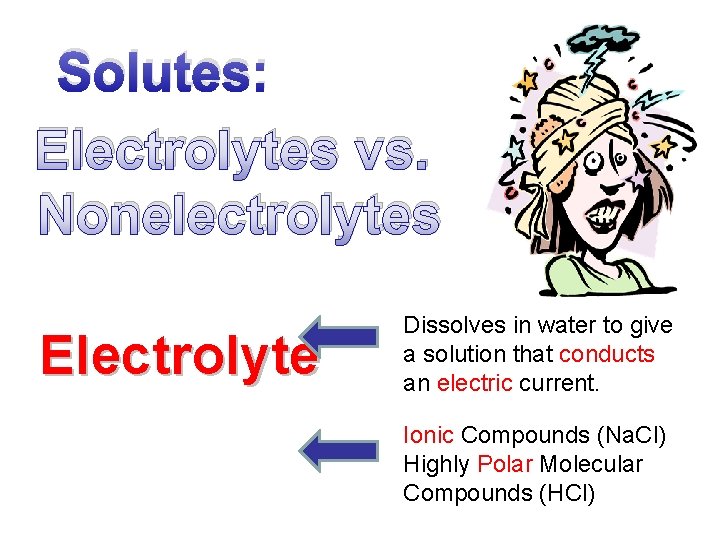
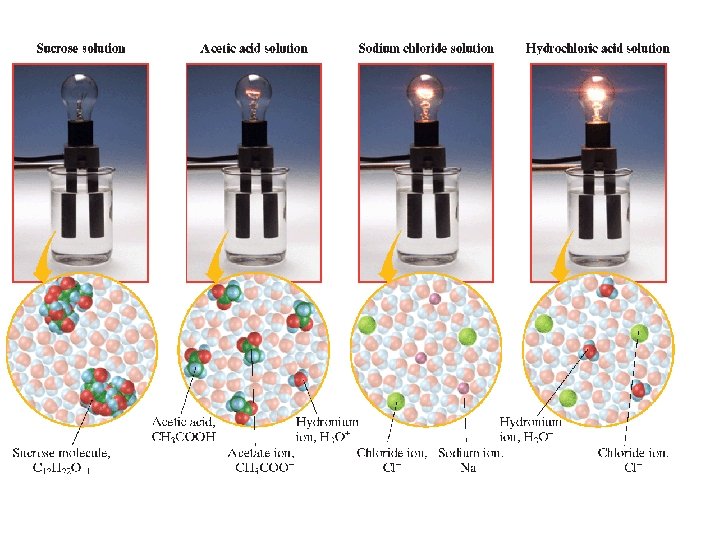
Solutes: Electrolytes vs. Nonelectrolytes Electrolyte Dissolves in water to give a solution that conducts an electric current. Ionic Compounds (Na. Cl) Highly Polar Molecular Compounds (HCl)

Nonelectrolyte Dissolves in water to give a solution that does not conduct electrical current. Neutral solute molecules (not charged particles). Sugar is a nonelectrolyte.


Questions: What does soluble mean? Is sugar more soluble in hot tea or iced tea?

Soluble By experience you know that sugar will dissolve in tea. Sugar is described as being “soluble” in tea. Soluble – capable of dissolving in a particular solvent.
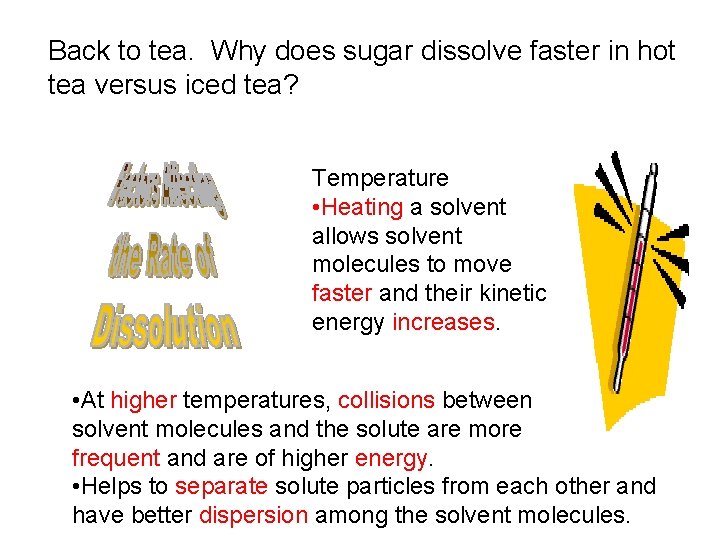
Back to tea. Why does sugar dissolve faster in hot tea versus iced tea? Temperature • Heating a solvent allows solvent molecules to move faster and their kinetic energy increases. • At higher temperatures, collisions between solvent molecules and the solute are more frequent and are of higher energy. • Helps to separate solute particles from each other and have better dispersion among the solvent molecules.
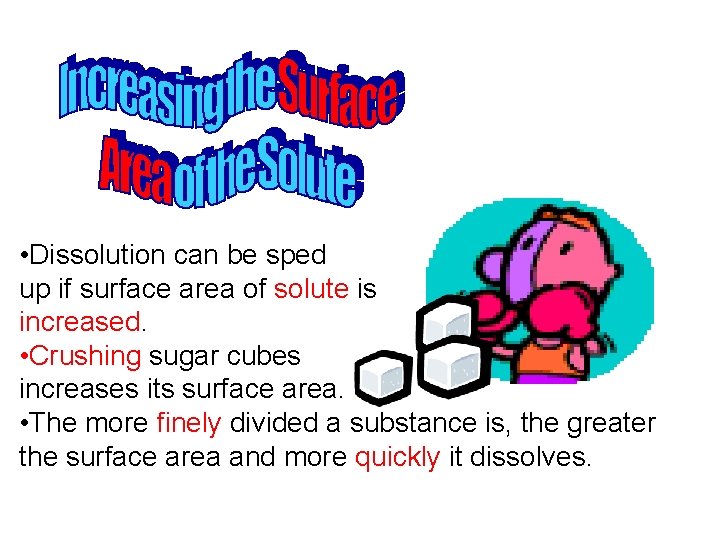
• Dissolution can be sped up if surface area of solute is increased. • Crushing sugar cubes increases its surface area. • The more finely divided a substance is, the greater the surface area and more quickly it dissolves.
• Stirring or shaking helps to disperse the solute particles and bring fresh solvent into contact with the solute surface. • Contact between the solute and solvent is increased.

Is there a limit to the amount of salt that will dissolve in a glass of water?
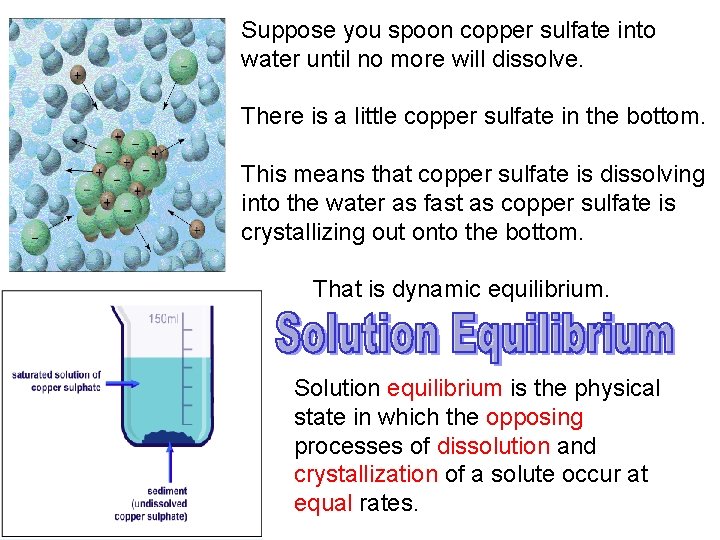
Suppose you spoon copper sulfate into water until no more will dissolve. There is a little copper sulfate in the bottom. This means that copper sulfate is dissolving into the water as fast as copper sulfate is crystallizing out onto the bottom. That is dynamic equilibrium. Solution equilibrium is the physical state in which the opposing processes of dissolution and crystallization of a solute occur at equal rates.
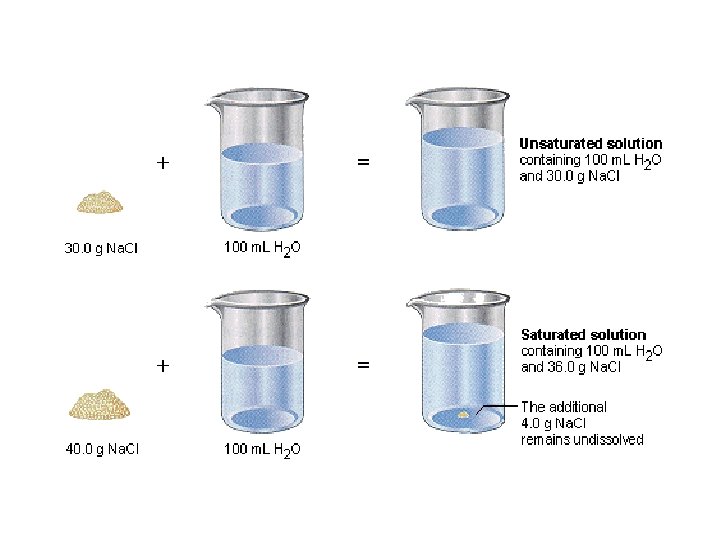
Saturated vs. Unsaturated A solution that contains the maximum amount of dissolved solute is described as a saturated solution. A solution that contains less solute than a saturated solution under the existing conditions is an unsaturated solution.

Supersaturated Solutio A supersaturated solution is a solution that contains more dissolved solute than a saturated solution contains under the same conditions. How does a supersaturated solution form? Heat saturated solution Let cool slowly – unstable If disturbed – will rapidly crystallize

Solubility The solubility of a substance is the amount of that substance required to form a saturated solution with a specific amount of solvent at a specified temperature.

Question: Why does vinegar dissolve in water but not in vegetable oil? Type of bonding, polarity or nonpolarity of molecules, and the intermolecular forces between the solute and the solvent.
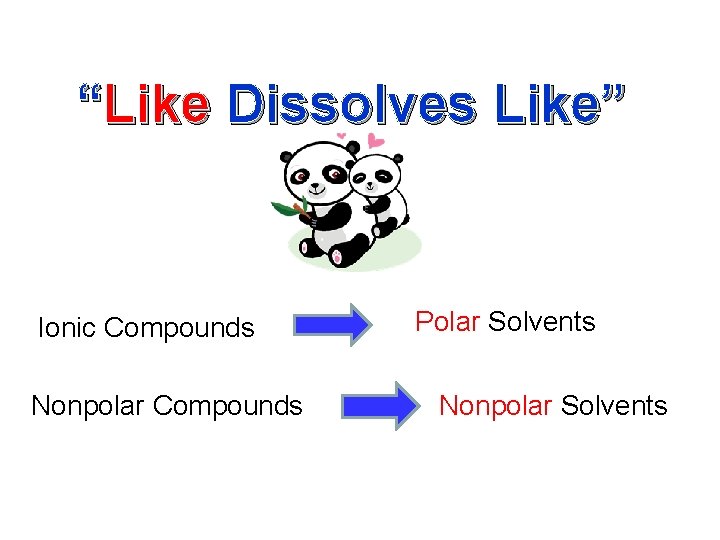
“Like Dissolves Like” Ionic Compounds Nonpolar Compounds Polar Solvents Nonpolar Solvents

Double, Toil and Trouble… • What can happen when we mix two liquids together? Either they mix, or they don’t! • If two liquids mix well together, and one dissolves in the other, we call them miscible (think “mixable”) • If two liquids do not mix well together, and they separate, we call them immiscible (think “unmixable”)

Like Oil & Water… • How do we know that two liquids will be miscible or immiscible? • Rule of thumb: “Like dissolves Like” – Polar solutes will dissolve in polar solvents. – Nonpolar solutes will dissolve in nonpolar solvents. • Water is the “universal solvent”.

…And never the two shall meet? • How can we mix polar and nonpolar molecules into solutions? Can it be done? • We use emulsifiers – agents that have both polar and nonpolar ends to join the two unlike molecules together. – Creates an emulsion: A suspension of small globules of one liquid in a second liquid with which the first will not mix.


The concentration of a solution is a measure of the amount of solute in a given amount of solvent or solution.
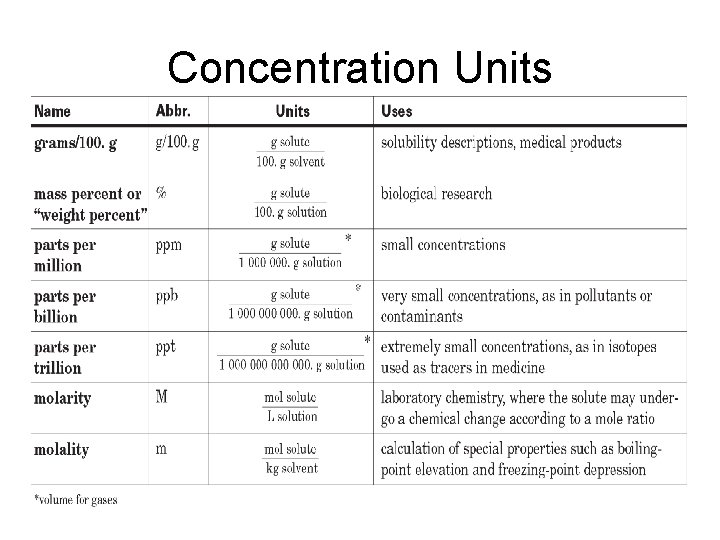
Concentration Units

Molarity is the number of moles of solute in one liter of solution. The symbol for molarity is M. For example, a “one molar” solution of sodium hydroxide, Na. OH, contains one mole of Na. OH in every liter of solution. The concentration of this solution would be written as 1 M Na. OH.

Note that a 1 M solution is not made by adding 1 mol of solute to 1 L of solvent.
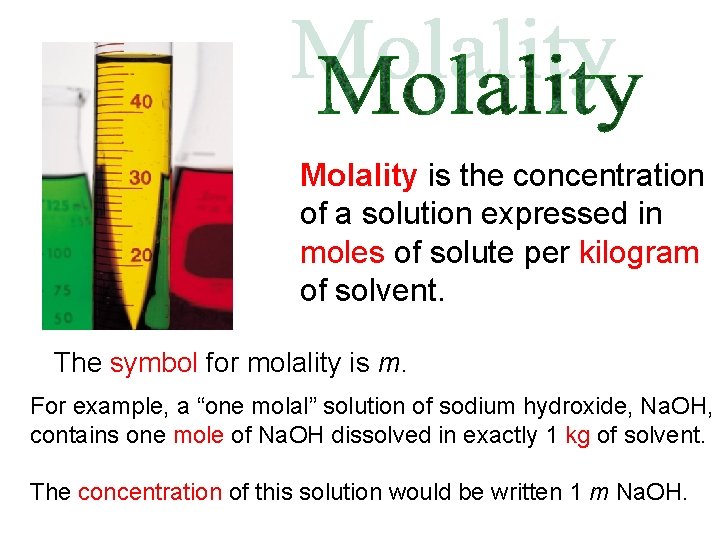
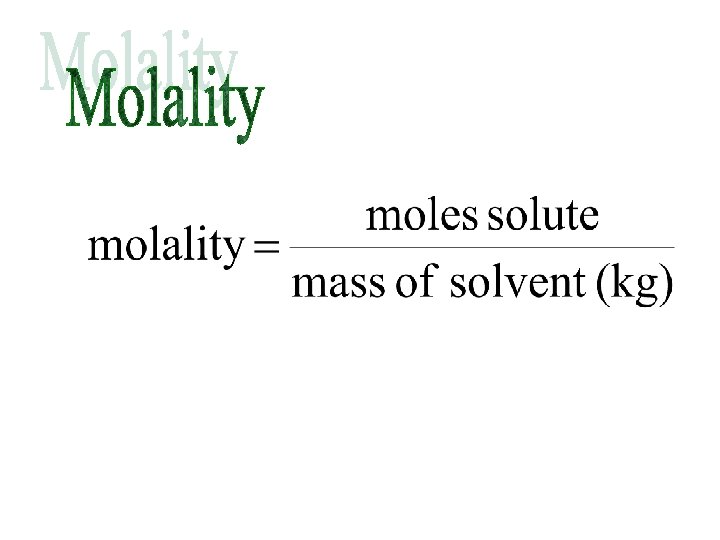
Molality is the concentration of a solution expressed in moles of solute per kilogram of solvent. The symbol for molality is m. For example, a “one molal” solution of sodium hydroxide, Na. OH, contains one mole of Na. OH dissolved in exactly 1 kg of solvent. The concentration of this solution would be written 1 m Na. OH.
- Slides: 36