A CLEARER PATH FOR PATHOGEN TESTING WHAT IS














































- Slides: 73

A CLEARER PATH FOR PATHOGEN TESTING


WHAT IS ACHIEVABLE IN PATHOGEN TESTING? Gary R. Acuff Professor, Food Microbiology Head, Department of Animal Science

Poll Question What is really achievable in pathogen reduction? 1. It depends upon how much contamination is present. 2. 3 Logs. 3. A scientific endpoint is needed. 4. Complete elimination of pathogens.

Pathogens ▶ ▶ Enterohemorrhagic Escherichia coli ▶ O 157: H 7 ▶ Non-O 157 Salmonella Campylobacter? Focus on EHECs

Source ▶ Cattle ▶ Gastrointestinal tract ▶ Anything in close proximity to feces ▶ Hide ▶ Hooves ▶ Dust ▶ Water

Control Opportunities ▶ ▶ ▶ Genomics Livestock production & feedlot ▶ Handling and well-being issues Slaughter Fabrication Retail Consumer, foodservice

Control Opportunities ▶ ▶ ▶ Genomics Livestock production & feedlot ▶ Handling and well-being issues Slaughter Fabrication Where are the hazards most effectively addressed? Retail Consumer, foodservice

Control Opportunities ▶ ▶ ▶ Genomics Livestock production & feedlot ▶ Handling and well-being issues Slaughter Fabrication Where are the hazards most effectively addressed? Retail Consumer, foodservice

Consumer Education

Carcass Interventions Temperature Chemical

Laboratory Challenge ►Parallel evaluations ►Marker pathogens in fecal material ►Salmonella serotype Typhimurium ►Escherichia coli O 157: H 7 ►Non-inoculated fecal material ►Indicator organisms Feces plus Pathogens

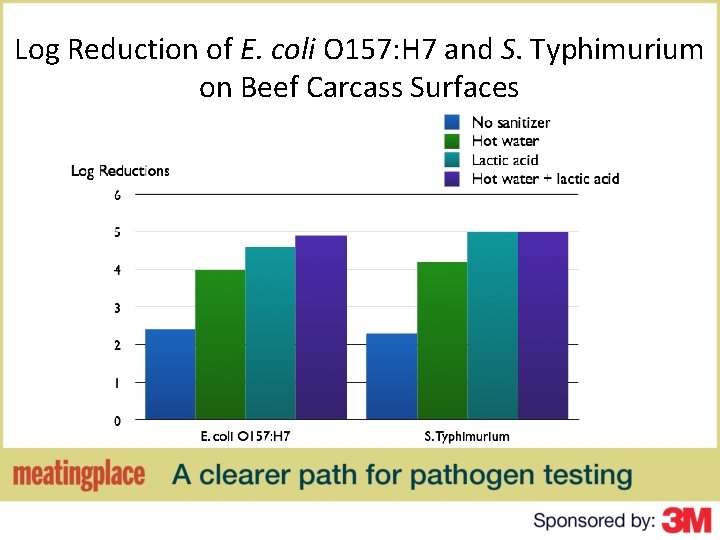
Log Reduction of E. coli O 157: H 7 and S. Typhimurium on Beef Carcass Surfaces

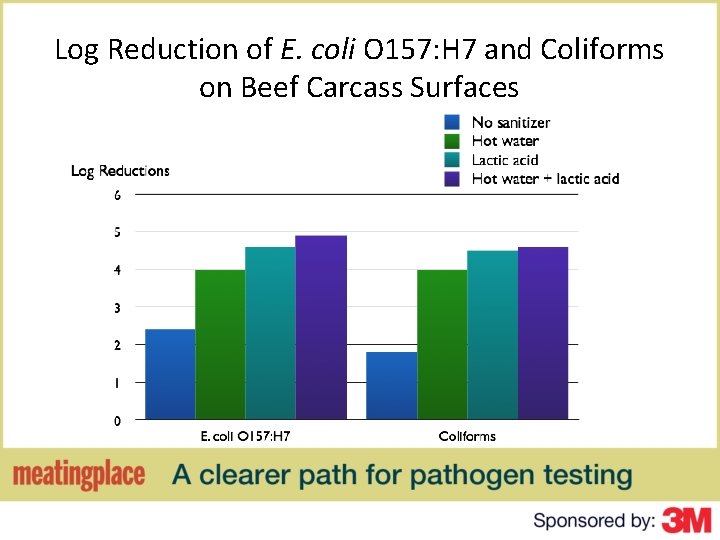
Log Reduction of E. coli O 157: H 7 and Coliforms on Beef Carcass Surfaces

What Is Realistic? ► Elimination? ► Reduction? ► The real issue: What remains? ► And where is it?






Trimming and grinding processes will distribute any remaining pathogens.

Unfortunate Event ▶ Adulterant declaration ▶ ▶ ▶ Well-intended (possibly), but short-sighted “Zero” doesn’t exist in bacterial enumeration Unintended consequence - difficult to measure improvement or control

What is really achievable? 1. 2. 3. 4. Does it really matter? It all has to be gone. Attempt to achieve what is required. Absence is unreasonable (impossible, illogical, unscientific, other favorite adjective).

An Endpoint is Needed ► How can one aim for a target that does not exist? ► Or one that constantly changes?

Food Safety Objective ▶ The maximum frequency and/or concentration of a hazard in a food at the time of consumption that provides or contributes to the appropriate level of protection.

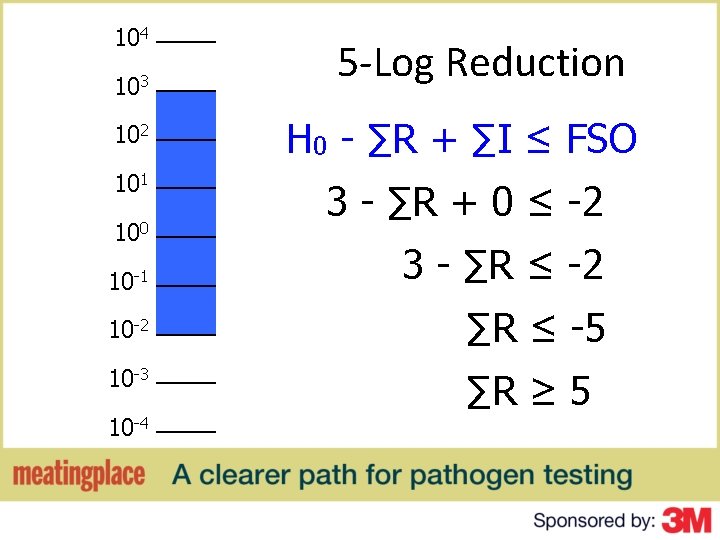
Food Safety Objective ▶ H 0 - ∑R + ∑I ≤ FSO ▶ ▶ ▶ FSO = Food Safety Objective H 0 = Initial level of the hazard ∑R = Total (cumulative) reduction of the hazard ∑I = Total (cumulative) increase of the hazard FSO, H 0, R and I are expressed in log 10 units

Food Safety Objective H 0 - ∑R + ∑I ≤ FSO 3 - ∑R + 0 ≤ -2 3 - ∑R ≤ -2 ∑R ≤ -5 ∑R ≥ 5

104 103 5 -Log Reduction 102 H 0 - ∑R + ∑I ≤ FSO 101 3 - ∑R + 0 ≤ -2 100 10 -1 3 - ∑R ≤ -2 10 -2 ∑R ≤ -5 10 -3 ∑R ≥ 5 10 -4

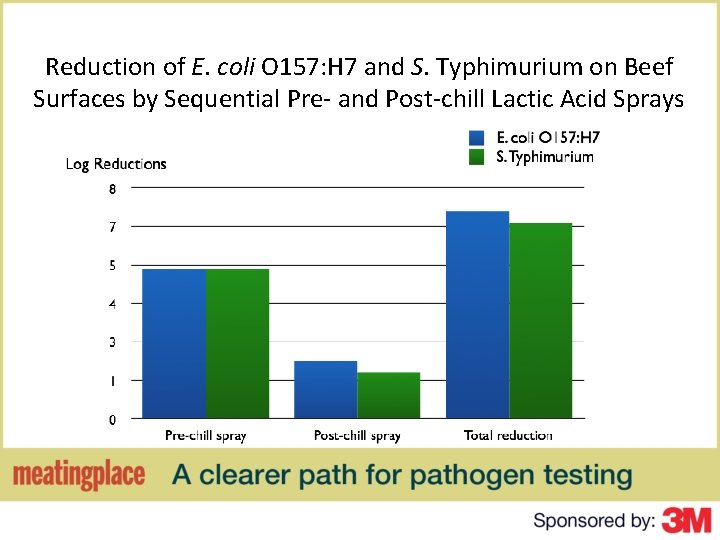
Reduction of E. coli O 157: H 7 and S. Typhimurium on Beef Surfaces by Sequential Pre- and Post-chill Lactic Acid Sprays

Microbiological Testing ▶ ▶ In the absence of an FSO, it is necessary to establish default criteria. “Safe Harbor”

Microbiological Testing Where are we going? ▶ Is there an end? ▶ Is more sampling the answer? ▶

Microbiological Testing ▶ In the ICMSF scheme for managing risk, two uses for criteria are identified. . . 1. To validate that control measures meet performance criteria 2. To determine acceptability when no more effective means of providing assurance is available (i. e. , in the absence of knowledge that HACCP has been properly applied)

Microbiological Testing ▶ If there is a way out, this may be it. In the ICMSF scheme for managing risk, two uses for criteria are identified. . . 1. To validate that control measures meet performance criteria 2. To determine acceptability when no more effective means of providing assurance is available (i. e. , in the absence of knowledge that HACCP has been properly applied)

Poll Question Results What is Really Achievable in Pathogen Reduction? 1. It depends upon how much contamination is present. 2. 3 Logs. 3. A scientific endpoint is needed. 4. Complete elimination of pathogens.

Provide a scientific endpoint and the industry will achieve the necessary reduction.

Food Safety At every step, 3 M Food Safety is dedicated to protecting your brand improving your productivity. Risk Reduction. Tools that provide trusted data for making informed decisions. Operational Efficiency. Reduce overtime and downtime - increase productivity. Competitive Edge. Solutions that create a powerful brand impact your bottom line. www. 3 M. com/foodsafety/MMW

CURRENT BEEF INDUSTRY SAMPLING AND TESTING PROGRAMS FOR E coli O 157: H 7 Timothy P. Biela EVP Food Safety & Quality AFA Foods

Poll Question Which best describes your position? Packer Processor Other Are you currently testing the following products for E coli O 157? Trim YES Ground Beef YES or or NO NO

What are we doing and why? • Carcass Testing – USDA Mandated carcass testing • Boneless Beef Trim Testing – N 60 Sampling and Testing – Sub-lot size varies from 1 to 5 combo bins • Raw Ground Beef Testing – Commercial versus Retail Testing Programs

Food Safety Verification for O 157 • Verification of HACCP and Food Safety Program for control of Enteric Pathogens – Must define the sublot – Must define the sample size and frequency of sampling – Must define the actions to be taken in the event of a positive result

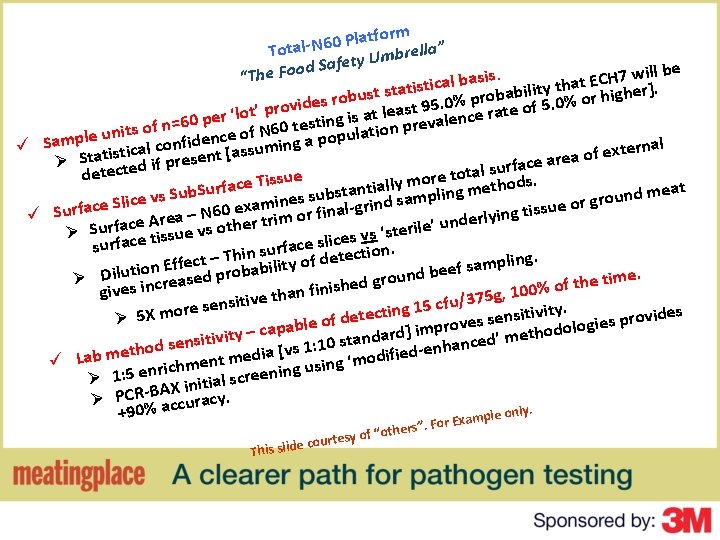
tform a l P 0 6 N Total rella” b m U y t e d Saf ill be . w s i 7 s “The Foo H a C b E l t atistica ity tha r higher]. t l i s b t a s b u o b r o ides r 5. 0% p rate of 5. 0% o v 9 o t r s p a ’ e t l o l t 0 per ‘ ng is a n prevalence i 6 t s = e n t f 0 o 6 s f. N nit atio l o u u e e p l c o p n p e m d a a i f ü S ternal x e tical conesent [assuming s f i t o a a t e S Ø if pr face ar r d u e t s c l e a t t e o d ore t ethods. issue T m e y l c l a a f i r t u n meat ub. S bsta d sampling m d S u n s s u v s o r e e c g n i i l ce S xam grin issue or t g n i y ü Surfa ace Area – N 60 heer trim or finall r ’ unde e l i r e t s ‘ Ø Surf ace tissue vs ot lices vs n. s e c a surf f r u – Thin s ility of detectio t ling. c p e f m f a E s n f b o e i a und be me. i o t Ø Dilust increased prob r e g h d t e f h o n finis 100% give a , h g t 5 7 e 3 v i / t i u g 15 cf re sens n o i t m c itivity. e s X t n 5 e ovides e r d s Ø p f s o s e e e v i l o g b r o rd] imp ced’ methodol y – capa a t i d v i n t a i t s s n nhan s 1: 10 e hod se v t [ d e a e i i m f d i b e d a o ü L ment m ning using ‘m h c i r n e 5 Ø 1: al scree i t i n i X A Ø PCR-B accuracy. nly. +90% xample o Thi rs”. For E e h t o “ f urtesy o s slide co

N 60 Sampling • 60 individual samples are taken from the lot • Preference is to sample surface material • Samples should be taken randomly across the entire “stream” or population of meat • Various sizes are used; 1 x 3, 2 x 5, etc…. • Lot sample size must be a minimum of 375 g • The entire sample should be analyzed

References • Beef Industry Food Safety Council Best Practices – http: //bifsco. org/harvest. aspx • USDA FSIS – http: //origin-www. fsis. usda. gov/PDF/Draft_Guidelines_Sampling_Beef_Trimmings_Ecoli. pdf • International Commission on Microbiological Specifications for Foods (ICMSF) – http: //www. icmsf. iit. edu/publications/sampling_plans. html

Microbiological Testing Programs must have: • A robust sampling plan that is designed to meet the microbiological testing objectives with a high degree of confidence. • The adequacy of the sampling plan should be evaluated by an independent third party. • In order to ensure the interpretability of testing results, the sampling plan must be implemented correctly. • Based on the results of microbiological testing, actions must be taken. (Plan / Do / Check / Act)

Key Elements of Sampling Plans • • Define what constitutes a sample, Define the number of samples to be collected, Define how samples will be collected, Define the number of samples it will collect, Define the frequency of sample collection, Define the procedures used to analyze samples and, Define the criteria for signaling an out-of-control process

Key Elements of Sampling Plans (2) • Ensure that samples collected are representative of the entire population; minimize bias, • Ensure that the sample size is sufficient to provide the desired level of confidence. • Ensure the sampling plan addresses the fact that pathogens are heterogeneously distributed • Ensure that sampling considers the variability in prevalence rates over time (seasonal variation).

Food Safety Verification for O 157 Example #1 • Major Retailer w Grinding Operation: – One sample every two hours of production. – Samples are analyzed with PCR/DNA. – Positive products are diverted to further processing. – Positives are bracketed from one hour in front of first positive to end of the day. – Positive rates range from. 4 to. 7%.

Food Safety Verification for O 157 Example #2 • Major Integrated Fed Beef Packer – Format varies from one sample per hour to one sample every fifteen minutes to screening preground products prior to packaging. – Samples are analyzed with PCR/DNA. – Positives are bracketed from one hour in front of first positive to one behind and subjected to intensified sampling. – Positive rates range from. 4 to. 7%.

Food Safety Verification for O 157 Example #3 • Major Quick Service Restaurant – One sample taken for every batch of ground beef formulated. – Samples are composited for four batches. – Composites are sampled and a 25 gram analyte is tested. – Positives are bracketed from one hour in front of first positive to end of the day. – Positive rates have ranged from 0 to. 2%.

HACCP and Risk Assessment for Raw Ground Beef Products • Retail versus Commercial Ground Beef – Retail • Consumers; Families • No identified platform for cooking • No verification of cooking controls – Commercial • Quick Serve and Casual Dining • Specific platforms for cooking and controls

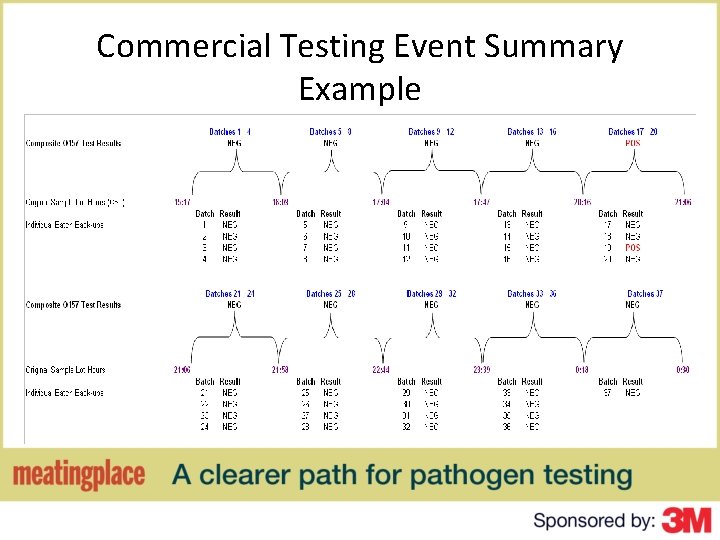
Commercial Testing Event Summary Example

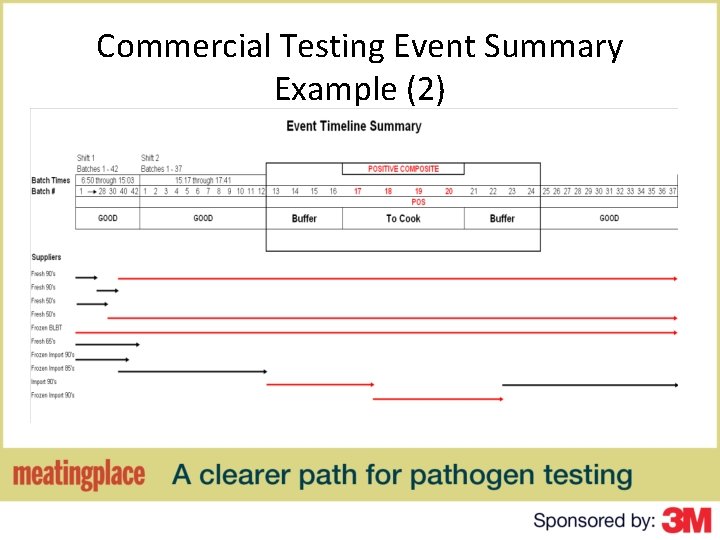
Commercial Testing Event Summary Example (2)

Summary Sampling and testing for pathogens is difficult. The key to an effective microbiological testing program is the sampling plan. Since it is not possible to conduct 100% testing, one must use a sample to draw inferences about the entire population. As indicated in the draft USDA Compliance Guideline, the sampling plan should be designed to provide a high probability of finding E. coli O 157: H 7, if it is present, and identifying a process that is out of control.

Summary (2) • • Due to the heterogeneous distribution of pathogens in meat products, microbiological testing cannot guarantee that product is free of contamination and should not be used for product acceptance. Microbiological testing should be used to verify process control and should occur on an ongoing basis as part of the “Plan-Do. Check-Act” cycle. Consistent and uniform sampling and testing methodologies for microbiological testing of beef manufacturing trimmings should be developed and implemented with the primary objective of protecting public health. N 60 sampling should be the minimum sampling protocol for beef manufacturing trimmings in all federally inspected meat and poultry establishments.

Poll Question Results Are you currently testing trim or raw ground beef products for E coli O 157? Trim Ground Beef Packer Processor YES YES or or NO NO NO No

BUILDING THE LABORATORY RELATIONSHIP Ted Brown Senior Food Scientist Cargill R&D Center

POLL QUESTION Lateral flow users: are you using a lateral flow or antigen/antibody based test that is specific for E. coli O 157: H 7? • Yes • No

Discussion Focus • Methodology Selection • Laboratory / Establishment Relationship

Before you Decide that Testing is necessary and on a Methodology Remember: Testing System is a PART of a Food Safety People: Knowledgeable & Skilled Processes & Programs Food Safety System Monitoring & Verification Investment

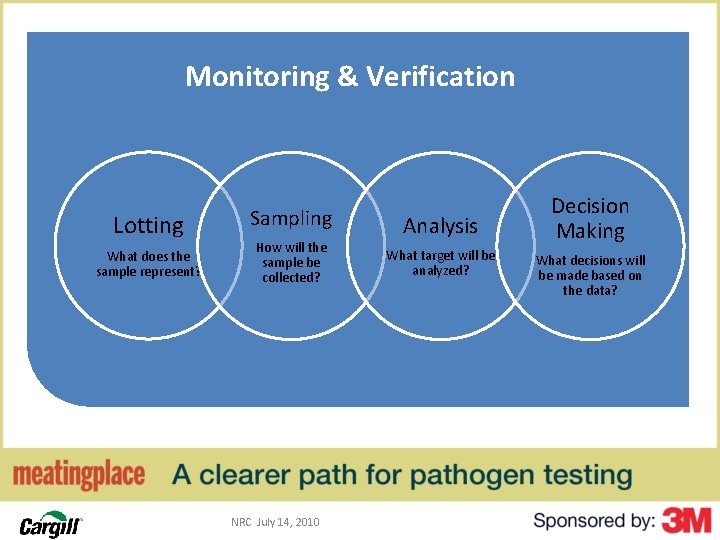
Monitoring & Verification Lotting What does the sample represent? 60 Sampling How will the sample be collected? NRC July 14, 2010 Analysis What target will be analyzed? Decision Making What decisions will be made based on the data?

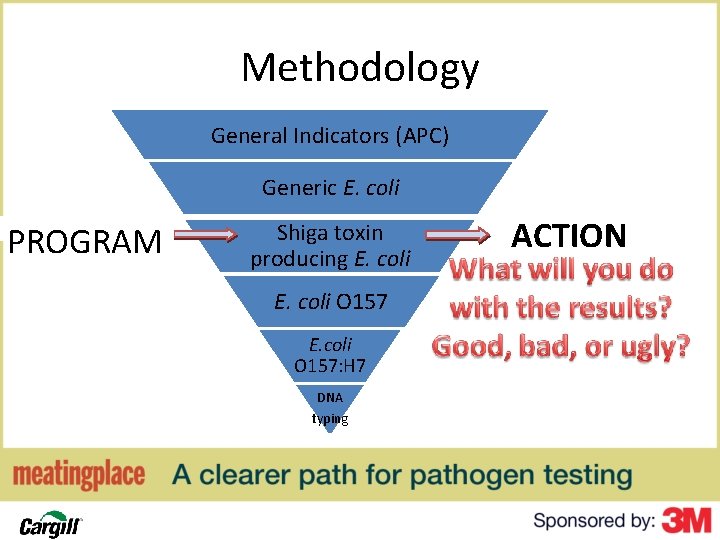
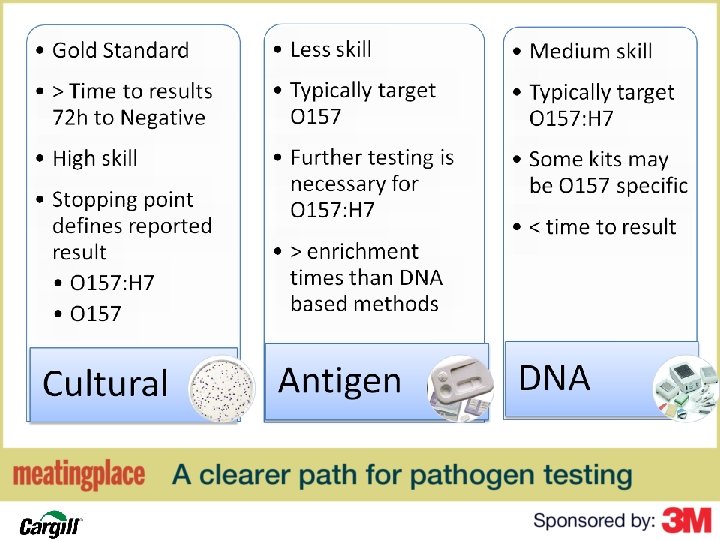
Methodology General Indicators (APC) Generic E. coli PROGRAM Shiga toxin producing E. coli O 157: H 7 DNA typing ACTION

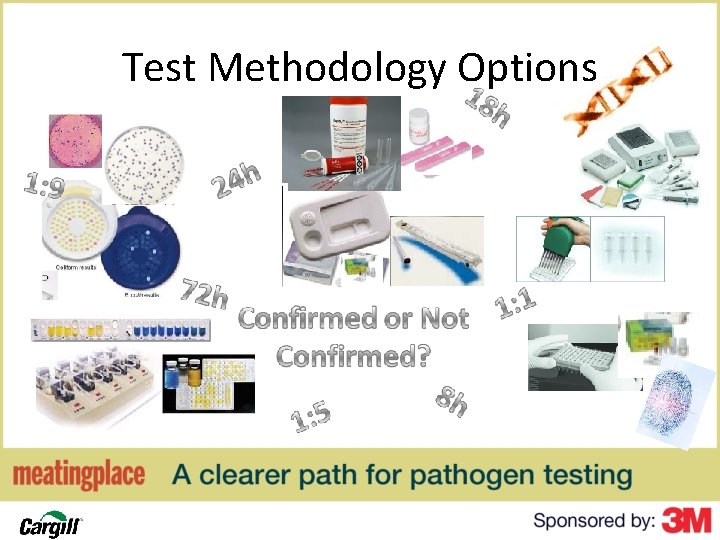
Test Methodology Options


Basis for Method Selection…

Test Method Application • Will the method provide the information needed to support my program objectives? • Do you know if the reported result for your method is O 157 or O 157: H 7 or something entirely different?

Approvals • Methods should be evaluated by an independent body • Not all approvals are created equal • Approval alone should not be the basis to make a method selection

Business Based Considerations • Goal is to find the target bug • This is not just a price based decision • This is an organization/business specific question with specific organization/business based answers

Fit for Use • Do I have the right tool for the job? – Is my method validated for the matrix as I am going to use it • Analytical unit size • Enrichment/Incubation • Detection limit • Are there any variations from the method as it was validated compared to my use?

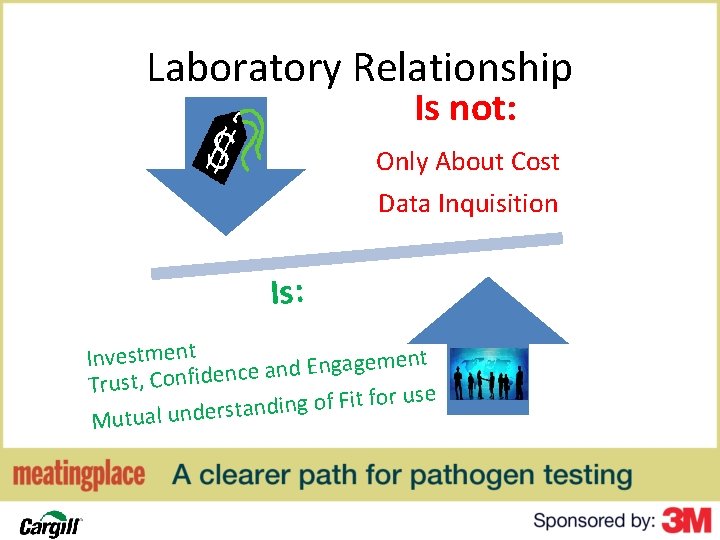
Laboratory Relationship Is not: Only About Cost Data Inquisition Is: Investment m e g a g n E d n a e c Trust, Confiden r use o f it F f o g in d n a Mutual underst

A CLEARER PATH FOR PATHOGEN TESTING • Testing is a PART of a Food Safety System. • Not all methods are created equal: O 157 positive result, does not mean that it is O 157: H 7 specifically • Who or what will the results effect? What will you do with the results? Good, bad, or Ugly? • Method must be fit for use when selected and as used • A lab relationship is an investment

POLL QUESTION RESULTS Lateral flow users: are you using a lateral flow or antigen/antibody based test that is specific for E. coli O 157: H 7? • Yes • No

QUESTIONS & ANSWERS

FOR MORE INFORMATION: Gary Acuff: gacuff@tamu. edu Timothy Biela: tbiela@amerfood. com Ted Brown: Ted_Brown@cargill. com 3 M: Christine Aleski , Global Marketing Development Manager email: cmaleski@mmm. com Bill Mc. Dowell: bmcdowell@meatingplace. com Tom Johnston: tjohnston@meatingplace. com Webinar recording and Power. Point presentation will be emailed to you within 48 hours. For more information: www. meatingplace. com/webinars