A chemical reaction is a change in composition
















- Slides: 28


A chemical reaction is a change in composition. • Is represented by a chemical equation which uses symbols and formulas. • Reactant(s)-the original substance(s) undergoing change • Product(s)- the new substance(s) produced A silver spoon tarnishes. The silver reacts with sulfur in the air to make silver sulfide, the black material we call tarnish. 2 Ag + S Ag 2 S Reactants Product

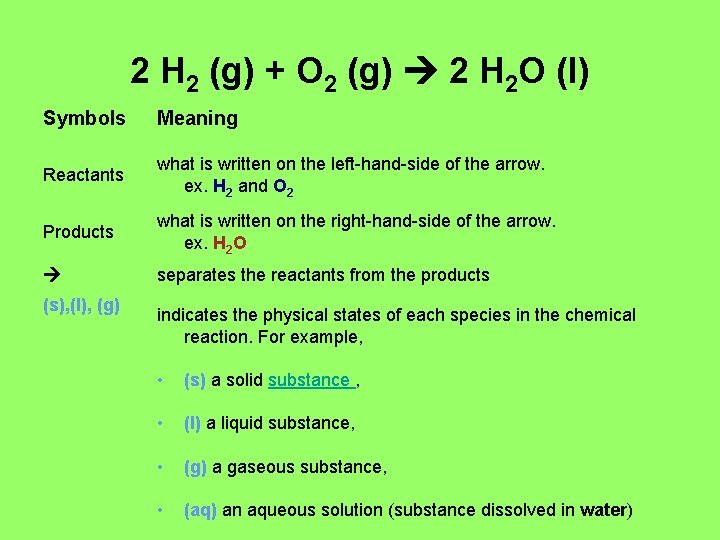
2 H 2 (g) + O 2 (g) 2 H 2 O (l) Symbols Meaning Reactants what is written on the left-hand-side of the arrow. ex. H 2 and O 2 Products what is written on the right-hand-side of the arrow. ex. H 2 O separates the reactants from the products (s), (l), (g) indicates the physical states of each species in the chemical reaction. For example, • (s) a solid substance , • (l) a liquid substance, • (g) a gaseous substance, • (aq) an aqueous solution (substance dissolved in water)

Types of Reactions • • • Synthesis Decomposition Single Displacement Double Displacement Combustion

SYNTHESIS REACTIONS ALSO CALLED COMBINATION, CONSTRUCTION, OR COMPOSITION REACTIONS • A + B AB • Two materials, elements or compounds, come together to make a single product. Some examples of synthesis reactions are: • Hydrogen gas and oxygen gas burn to produce water. 2 H 2 + O 2 2 H 2 O • sulfur trioxide reacts with water to make sulfuric acid. H 2 O + SO 3 H 2 SO 4

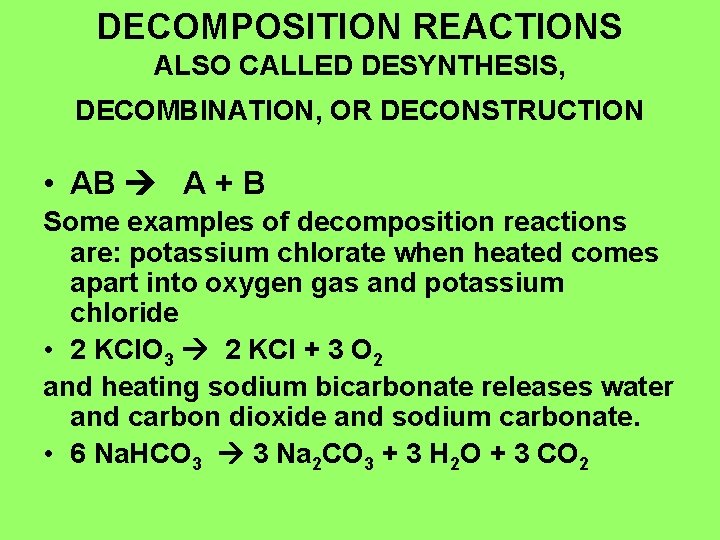
DECOMPOSITION REACTIONS ALSO CALLED DESYNTHESIS, DECOMBINATION, OR DECONSTRUCTION • AB A + B Some examples of decomposition reactions are: potassium chlorate when heated comes apart into oxygen gas and potassium chloride • 2 KCl. O 3 2 KCl + 3 O 2 and heating sodium bicarbonate releases water and carbon dioxide and sodium carbonate. • 6 Na. HCO 3 3 Na 2 CO 3 + 3 H 2 O + 3 CO 2

SINGLE REPLACEMENT REACTIONS ALSO CALLED SINGLE DISPLACEMENT, SINGLE SUBSTITUTION, OR ACTIVITY REPLACEMENT • 2 KI + Cl 2 2 KCl + I 2 • Positive ions will replace positive ionsnegative ions will replace negative ions

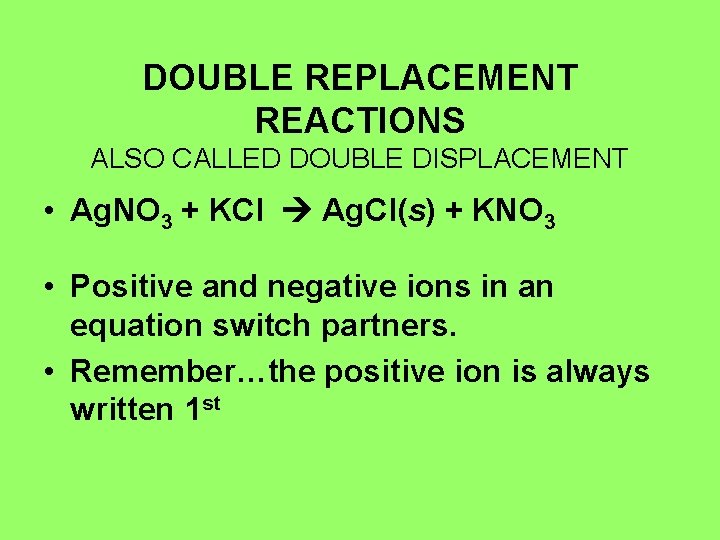
DOUBLE REPLACEMENT REACTIONS ALSO CALLED DOUBLE DISPLACEMENT • Ag. NO 3 + KCl Ag. Cl(s) + KNO 3 • Positive and negative ions in an equation switch partners. • Remember…the positive ion is always written 1 st

Combustion Reactions • Occurs between a hydrocarbon and oxygen • Products are always carbon dioxide and water • CH 4 +2 O 2 CO 2 + 2 H 2 O

• The products of a reaction are always made up of the same number and kinds of atoms. • The law of conservation of mass is satisfied by balancing chemical equations.

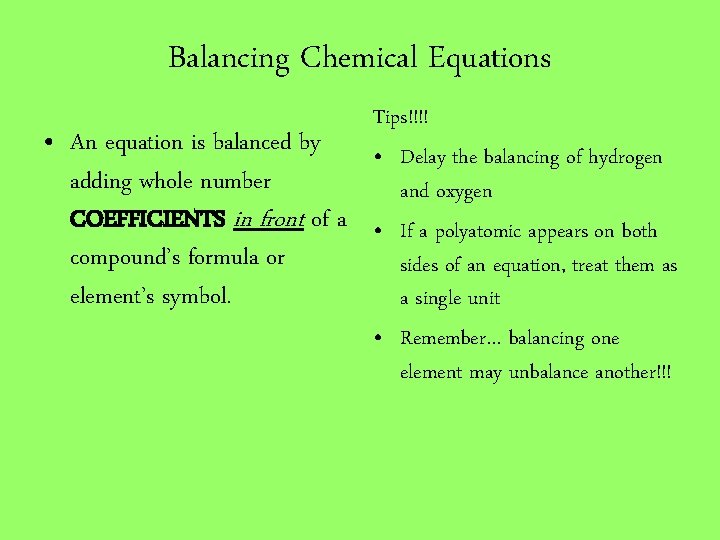
Balancing Chemical Equations Tips!!!! • An equation is balanced by • Delay the balancing of hydrogen adding whole number and oxygen COEFFICIENTS in front of a • If a polyatomic appears on both compound’s formula or sides of an equation, treat them as element’s symbol. a single unit • Remember… balancing one element may unbalance another!!!

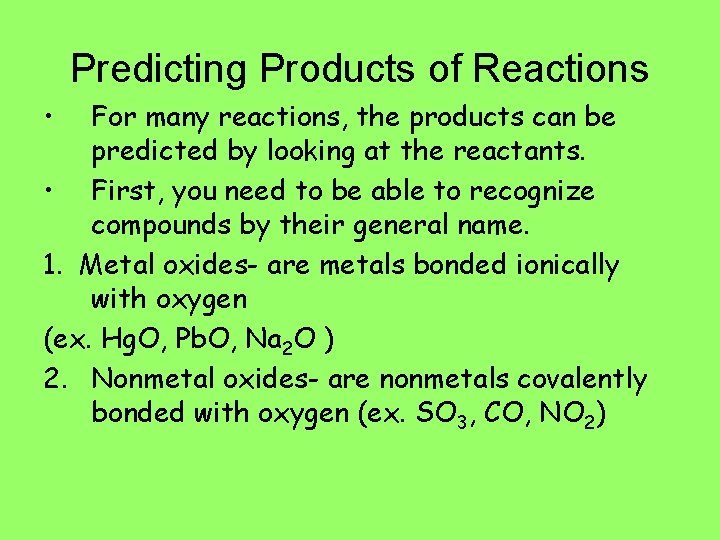
Predicting Products of Reactions • For many reactions, the products can be predicted by looking at the reactants. • First, you need to be able to recognize compounds by their general name. 1. Metal oxides- are metals bonded ionically with oxygen (ex. Hg. O, Pb. O, Na 2 O ) 2. Nonmetal oxides- are nonmetals covalently bonded with oxygen (ex. SO 3, CO, NO 2)

3. Binary Compounds- are compounds with only two elements (ex. Na. I, Mg. Cl 2) 4. Metal Carbonates- are metals bonded with the polyatomic carbonate (ex. Ca. CO 3, Na 2 CO 3, Al 2(CO 3)3 ) 5. Metal Hydroxides- are metals bonded with the polyatomic hydroxide (ex. Mg(OH)2, Na. OH ) 6. Metal Chlorates- are metals bonded with the polyatomic chlorate (ex. KCl. O 3, Mg(Cl. O 3)2 )

7. Metal Chlorides- metals bonded with the element chlorine (ex. Na. Cl, Mg. Cl 2 ) 8. Hydrocarbons- are covalent bonds formed only from hydrogen and carbon (ex. C 3 H 8, CH 4, C 2 H 6)


F 2 Cl 2 Br 2 H 2 I 2 N 2 O 2


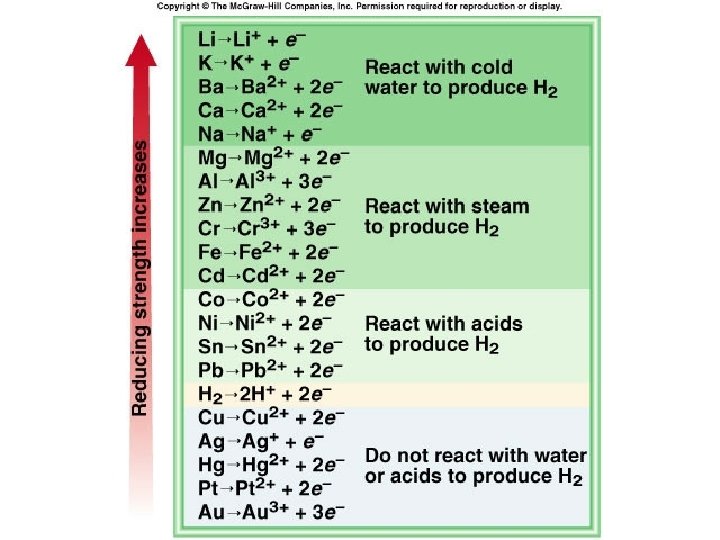
Activity- the ability of an element to react • Activity Series- a list • The most active of elements organized elements are at the according to the ease top-the least reactive with which they at the bottom undergo single • Elements can replacement reactions those that are below them (i. e. those that are less reactive) but not those above them (i. e. those that are more reactive)

• Each element on the list replaces from a compound any of the elements below it. The larger the interval between elements, the more vigorous the reaction. • The first seven elements (lithium - sodium) are known as very active metals and they react with cold water to produce the hydroxide and hydrogen gas. • The next seven metals (magnesium - cadmium) are considered active metals and they will react with very hot water or steam to form the oxide and hydrogen gas. • The oxides of metals above Fe of these first metals resist decomposition by heating with H 2. • The next four metals (cobalt - lead) replace hydrogen from HCl and dil. sulfuric and nitric acids.

• Oxides of metals below chromium decompose with heat alone. • The metals hydrogen - copper, can combine directly with oxygen to form the oxide. • The last five metals (mercury - gold) are often found free in nature, their oxides decompose with mild heating, and they form oxides only indirectly • Groups can do anything listed in their column and anything below them but they can never do things listed above them. (For instance, Li can react with cold water, steam, acids, and oxygen. Pb can react with acids and oxygen but not cold water or steam.

Examples: • Al + Zn. Cl 2 This reaction would go to completion because aluminum is above zinc in the series and can therefore replace it. • Co + Na. Cl NR Cobalt can not replace Na because Na is above it in the series and is therefore the more reactive element.

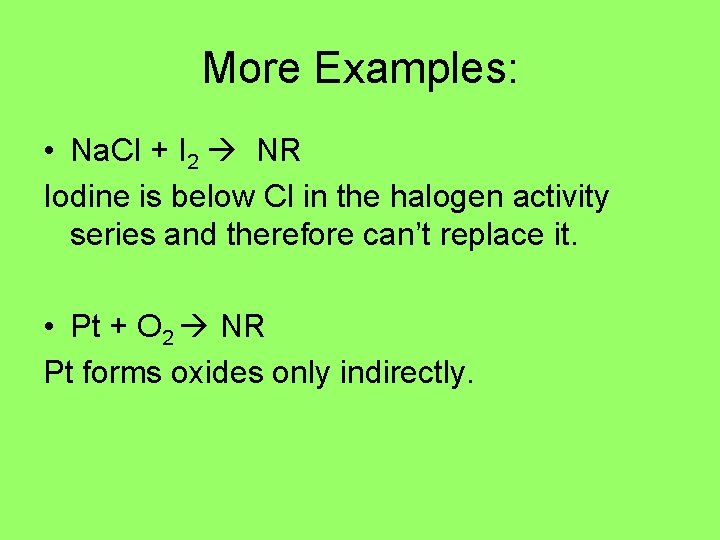
More Examples: • Na. Cl + I 2 NR Iodine is below Cl in the halogen activity series and therefore can’t replace it. • Pt + O 2 NR Pt forms oxides only indirectly.

More Examples: • Al + H 2 O (l) NR Al can only react with water that is steam (a. k. a. gas) • Li + HCl This reaction would go to completion because Li can react with acids (remember. . they can do anything in their group and the groups below them)

More Examples: • Mg. O NR Metal oxides above Fe do not decompose with heat. • Fe. O (heated) This reaction would go to completion since it was heated.


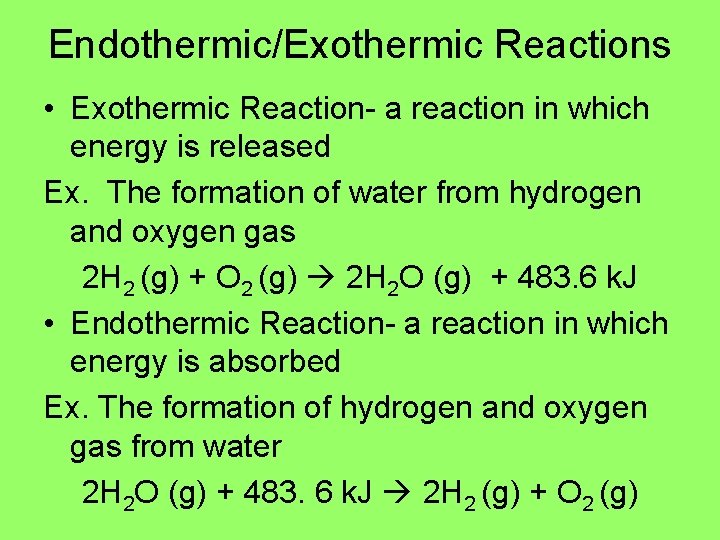
Endothermic/Exothermic Reactions • Exothermic Reaction- a reaction in which energy is released Ex. The formation of water from hydrogen and oxygen gas 2 H 2 (g) + O 2 (g) 2 H 2 O (g) + 483. 6 k. J • Endothermic Reaction- a reaction in which energy is absorbed Ex. The formation of hydrogen and oxygen gas from water 2 H 2 O (g) + 483. 6 k. J 2 H 2 (g) + O 2 (g)

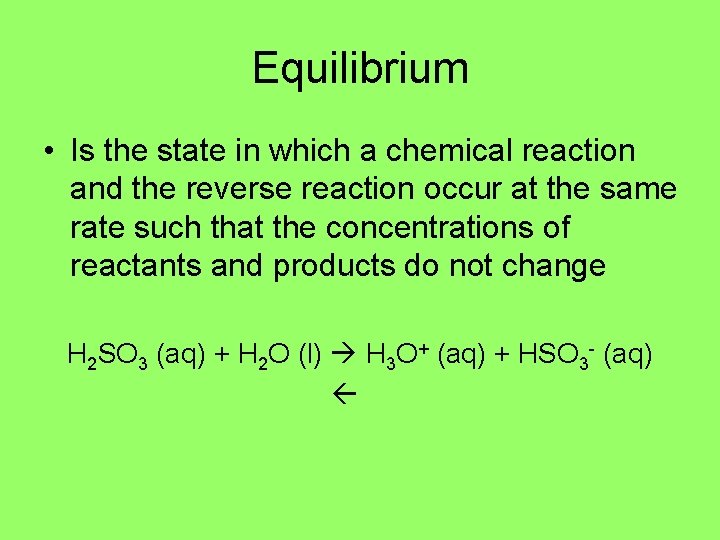
Equilibrium • Is the state in which a chemical reaction and the reverse reaction occur at the same rate such that the concentrations of reactants and products do not change H 2 SO 3 (aq) + H 2 O (l) H 3 O+ (aq) + HSO 3 - (aq)

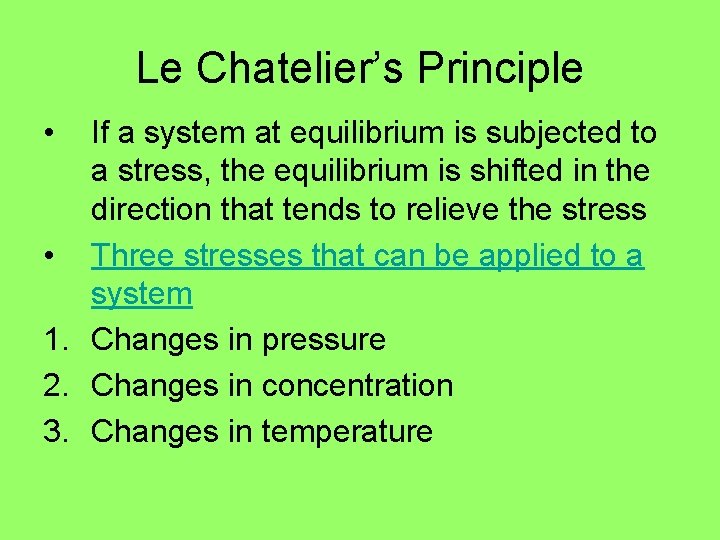
Le Chatelier’s Principle • If a system at equilibrium is subjected to a stress, the equilibrium is shifted in the direction that tends to relieve the stress • Three stresses that can be applied to a system 1. Changes in pressure 2. Changes in concentration 3. Changes in temperature