A Brief History leading up to Rutherford Democritus

A Brief History leading up to Rutherford

Democritus Model (> 2400 years ago) Do things come from nothing? • • • Everything is made up of atoms. from the Greek adjective atomos or atomon, ‘indivisible, ’ All changes in the visible objects of the world of appearance are brought about by relocations of these atoms: Could matter be divided into smaller and smaller pieces forever, or was there a limit to the number of times a piece of matter could be divided? • Atoms are the smallest pieces.
Democritus Model (> 2400 years ago) • Indivisible and indestructible • An infinite number of atoms and kinds of atoms, which differ in shape, and size • Separated by empty space • Perfectly solid, with no internal gaps. • Have always been, and always will be, in motion • Repel one another when they collide or combine into clusters by means of tiny hooks and barbs on their surfaces
Dalton Model (> 200 years ago) • All atoms of a given element are identical in mass and properties. • Compounds are formed by a combination of two or more different kinds of atoms. • A chemical reaction is a rearrangement of atoms. • Atoms can be neither created nor destroyed – in a chemical reaction, or any time!
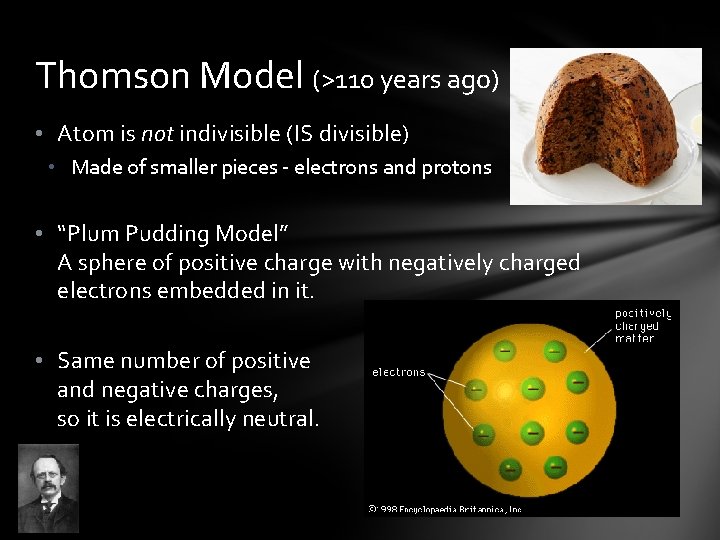
Thomson Model (>110 years ago) • Atom is not indivisible (IS divisible) • Made of smaller pieces - electrons and protons • “Plum Pudding Model” A sphere of positive charge with negatively charged electrons embedded in it. • Same number of positive and negative charges, so it is electrically neutral.
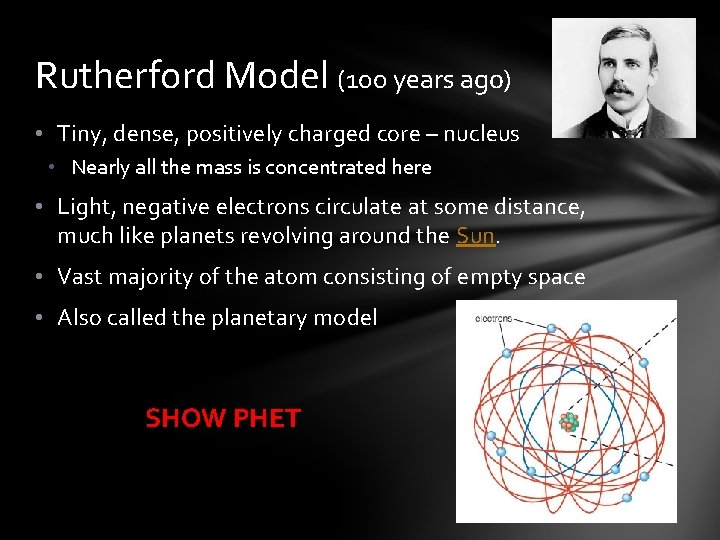
Rutherford Model (100 years ago) • Tiny, dense, positively charged core – nucleus • Nearly all the mass is concentrated here • Light, negative electrons circulate at some distance, much like planets revolving around the Sun. • Vast majority of the atom consisting of empty space • Also called the planetary model SHOW PHET
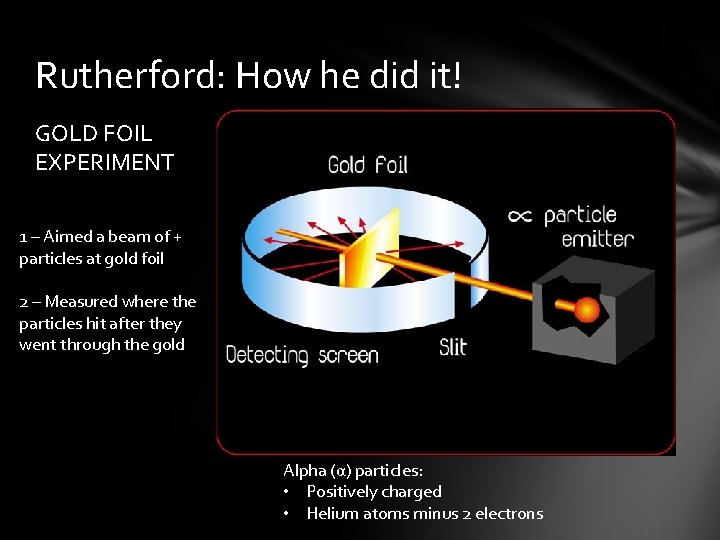
Rutherford: How he did it! GOLD FOIL EXPERIMENT 1 – Aimed a beam of + particles at gold foil 2 – Measured where the particles hit after they went through the gold Alpha (α) particles: • Positively charged • Helium atoms minus 2 electrons
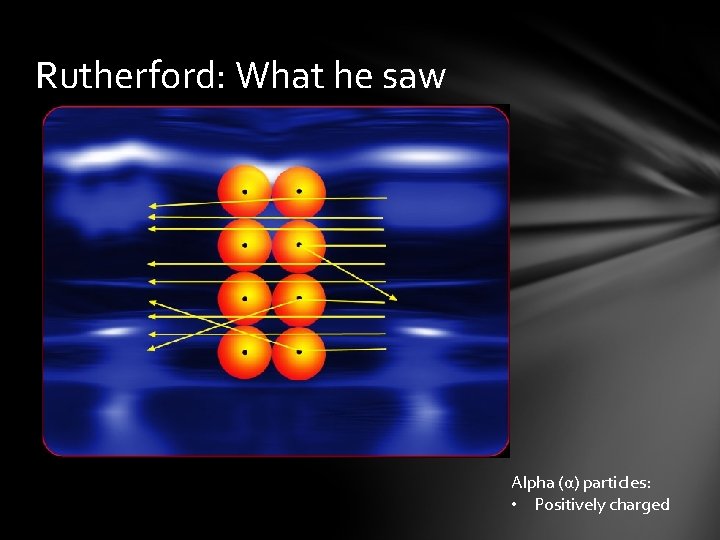
Rutherford: What he saw Alpha (α) particles: • Positively charged
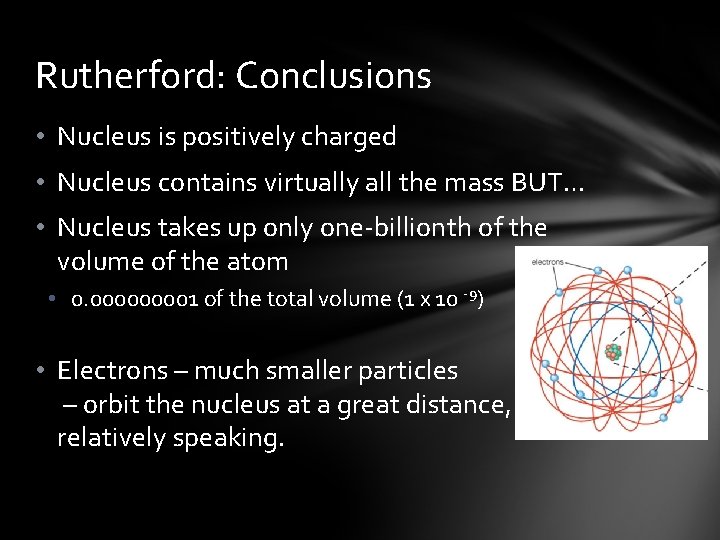
Rutherford: Conclusions • Nucleus is positively charged • Nucleus contains virtually all the mass BUT… • Nucleus takes up only one-billionth of the volume of the atom • 0. 00001 of the total volume (1 x 10 -9) • Electrons – much smaller particles – orbit the nucleus at a great distance, relatively speaking.

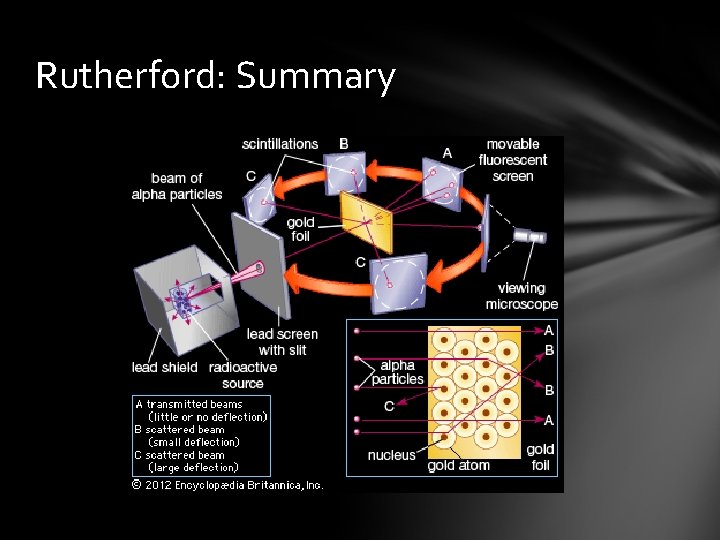
Rutherford: Summary

Democritus Model (> 2400 years ago) Reasoning: the solidness of the material corresponded to the shape of the atoms involved. Thus: • Iron atoms are solid and strong with hooks that lock them into a solid • Water atoms are smooth and slippery • Salt atoms, because of their taste, are sharp and pointed • Air atoms are light and whirling Show: Phet Simulation
Dalton Model (> 200 years ago) • All matter is made of atoms. Atoms are indivisible and indestructible. • All atoms of a given element are identical in mass and properties. • Compounds are formed by a combination of two or more different kinds of atoms. • A chemical reaction is a rearrangement of atoms. • Atoms can be neither created nor destroyed – in a chemical reaction, or any time!
- Slides: 13