a beta gamma alpha and RADIATION Marie Curie

a beta gamma alpha α, and RADIATION Marie Curie Antoine-Henri Becquerel (1852 – 1908) © JP 1
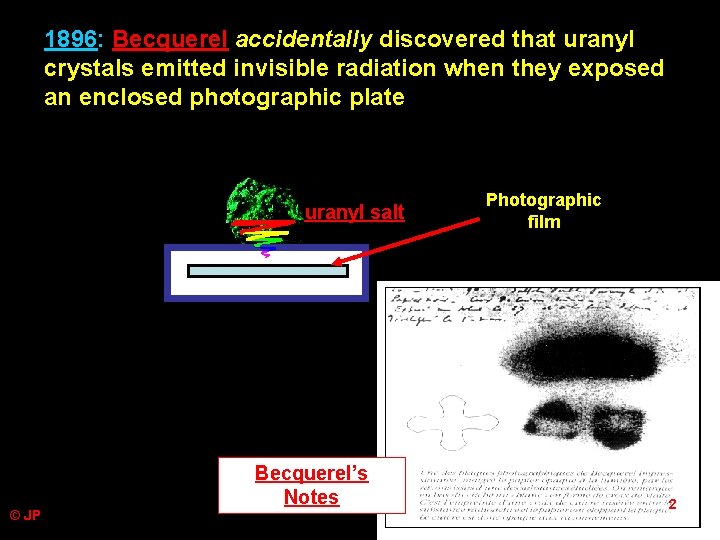
1896: Becquerel accidentally discovered that uranyl crystals emitted invisible radiation when they exposed an enclosed photographic plate uranyl salt © JP Becquerel’s Notes Photographic film 2

90 thorium Marie Curie discovered that thorium, (Z=90) was a radioactive element 1867 -1934 1898: Marie and Pierre Curie discovered polonium (Z=84) and radium (Z = 88), two new radioactive elements 84 polonium © JP radium 88 as paint 3
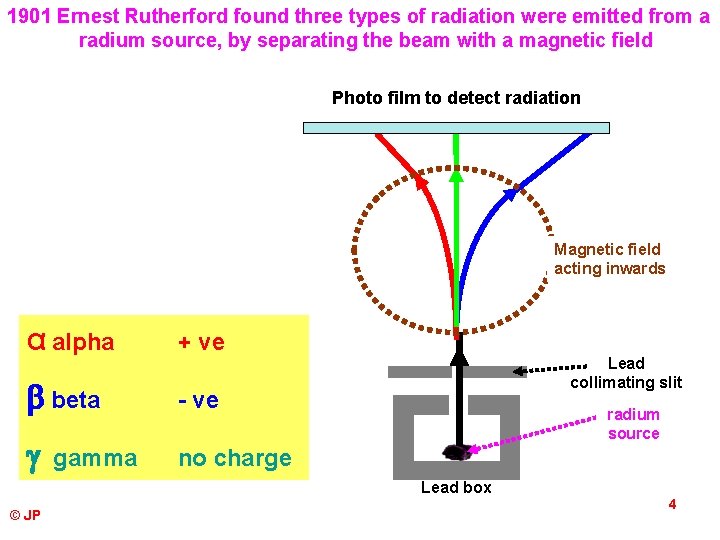
1901 Ernest Rutherford found three types of radiation were emitted from a radium source, by separating the beam with a magnetic field Photo film to detect radiation Magnetic field acting inwards α alpha + ve beta - ve no charge gamma Lead collimating slit radium source Lead box © JP 4
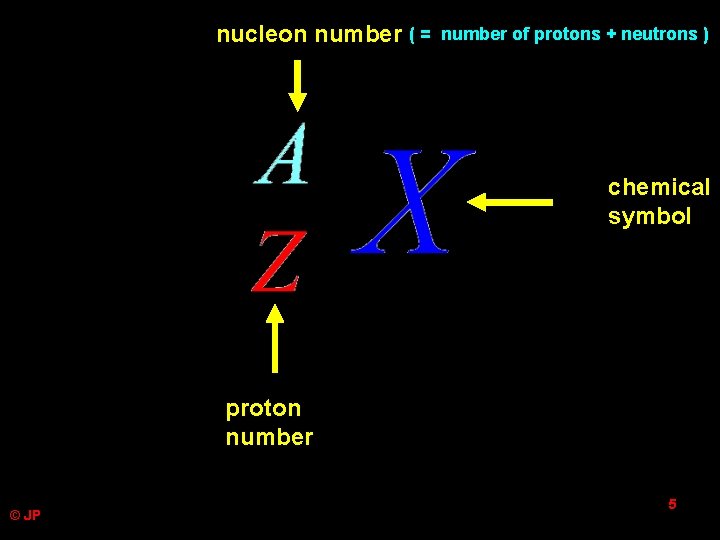
nucleon number ( = number of protons + neutrons ) chemical symbol proton number © JP 5

Alpha particles are helium nuclei Typical speed 0. 1 c; energy 5 Me. V Beta particles are high speed electrons Typical speed 0. 99 c Gamma rays are energetic photons speed = c ; λ = 10 -11 – 10 -13 m © JP 6
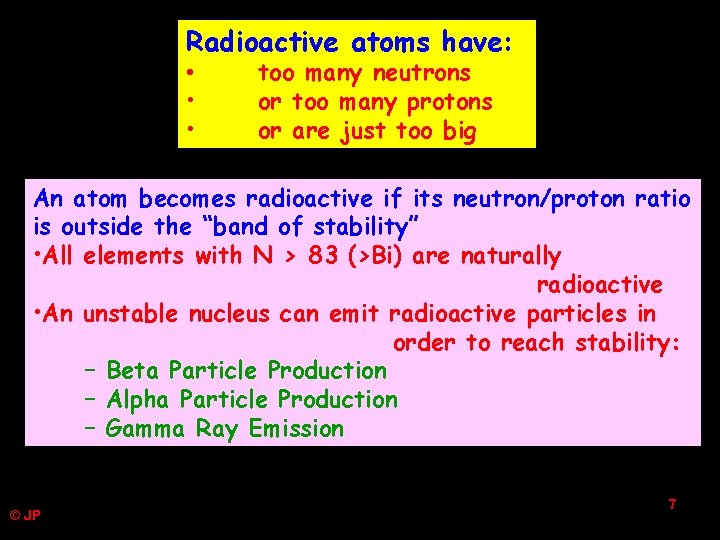
Radioactive atoms have: • • • too many neutrons or too many protons or are just too big An atom becomes radioactive if its neutron/proton ratio is outside the “band of stability” • All elements with N > 83 (>Bi) are naturally radioactive • An unstable nucleus can emit radioactive particles in order to reach stability: – Beta Particle Production – Alpha Particle Production – Gamma Ray Emission © JP 7
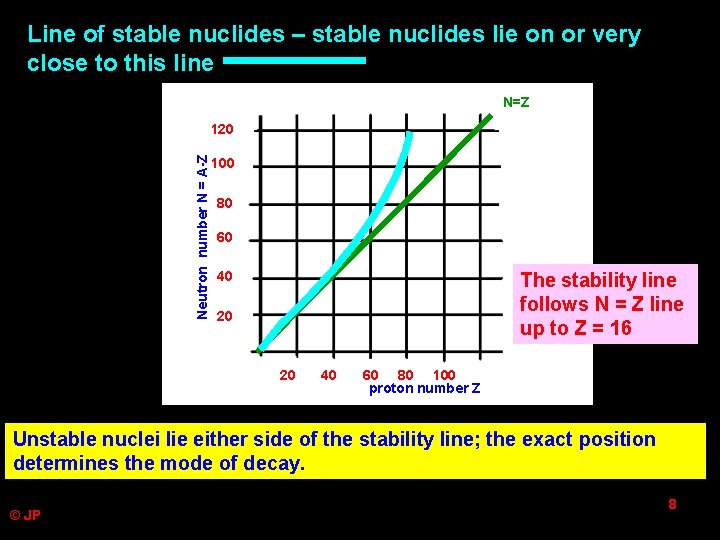
Line of stable nuclides – stable nuclides lie on or very close to this line N=Z Neutron number N = A-Z 120 100 80 60 40 The stability line follows N = Z line up to Z = 16 20 20 40 60 80 100 proton number Z Unstable nuclei lie either side of the stability line; the exact position determines the mode of decay. © JP 8
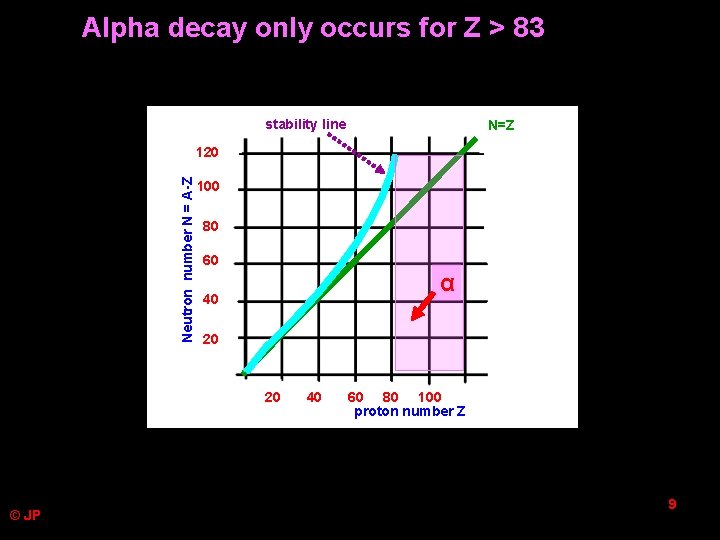
Alpha decay only occurs for Z > 83 stability line N=Z Neutron number N = A-Z 120 100 80 60 α 40 20 20 © JP 40 60 80 100 proton number Z 9
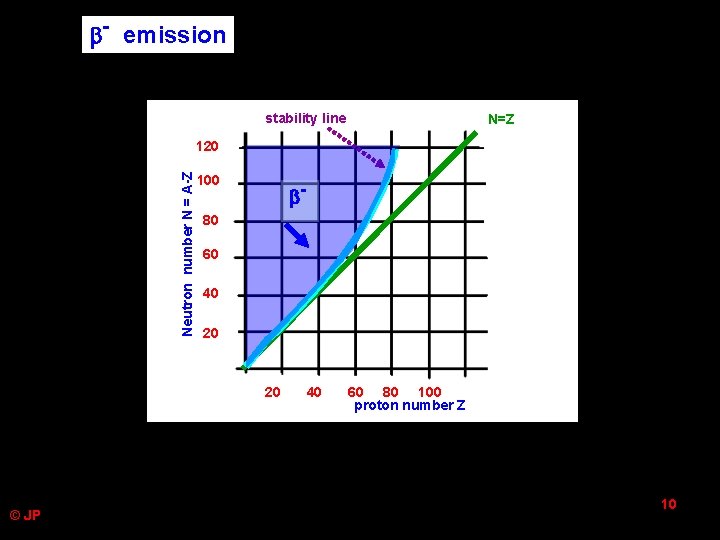
- emission stability line N=Z Neutron number N = A-Z 120 100 - 80 60 40 20 20 © JP 40 60 80 100 proton number Z 10
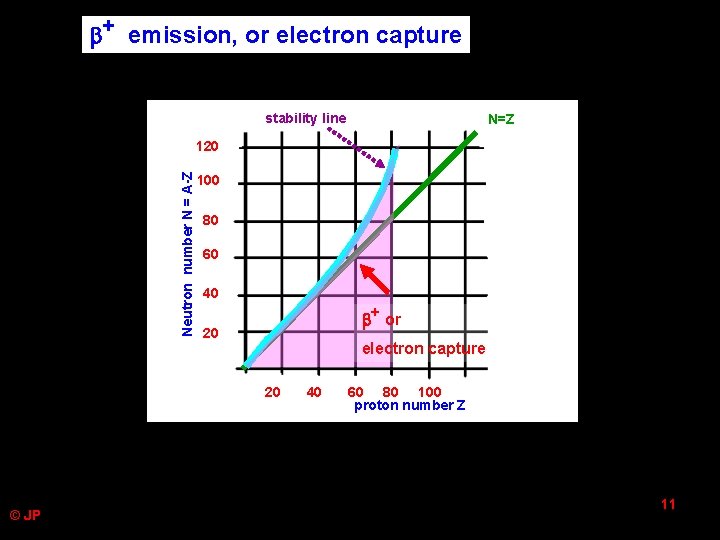
+ emission, or electron capture stability line N=Z Neutron number N = A-Z 120 100 80 60 40 + or 20 electron capture 20 © JP 40 60 80 100 proton number Z 11
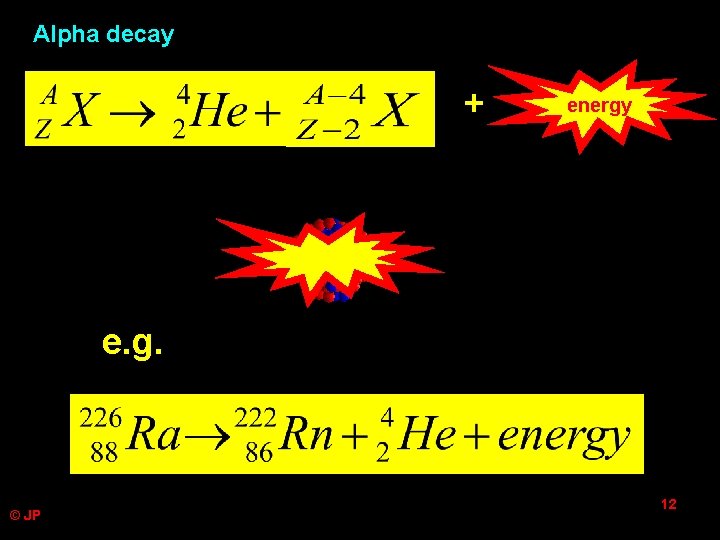
Alpha decay + ? energy e. g. © JP 12
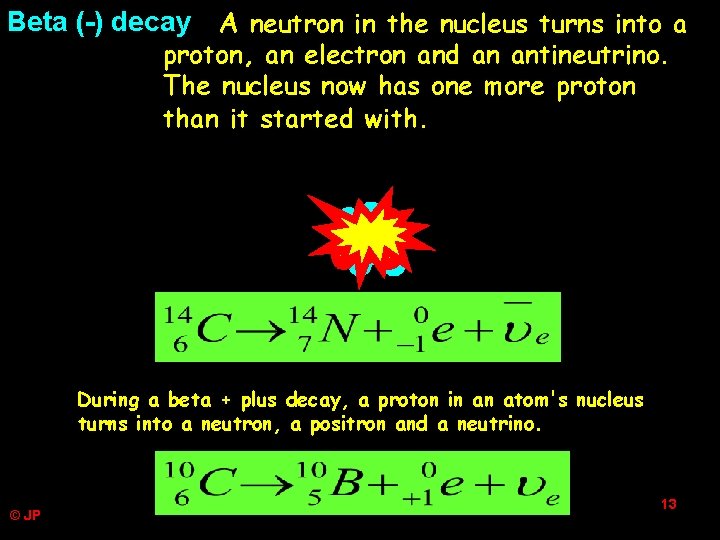
A neutron in the nucleus turns into a proton, an electron and an antineutrino. The nucleus now has one more proton than it started with. Beta (-) decay + + + During a beta + plus decay, a proton in an atom's nucleus turns into a neutron, a positron and a neutrino. © JP 13
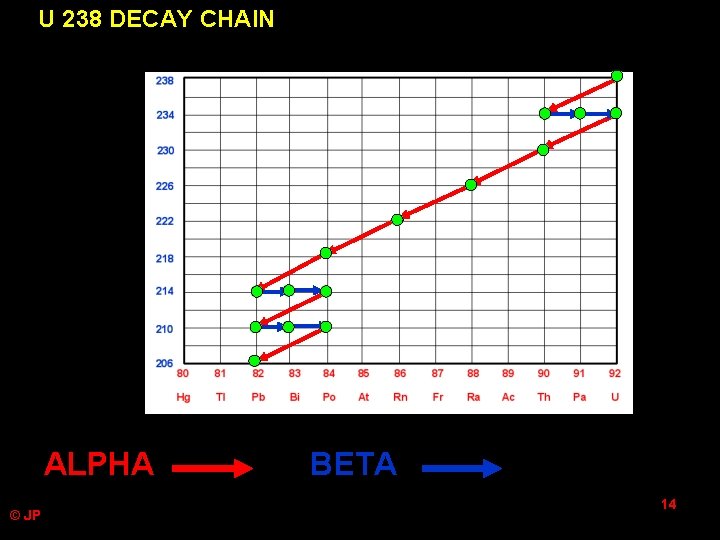
U 238 DECAY CHAIN ALPHA © JP BETA 14
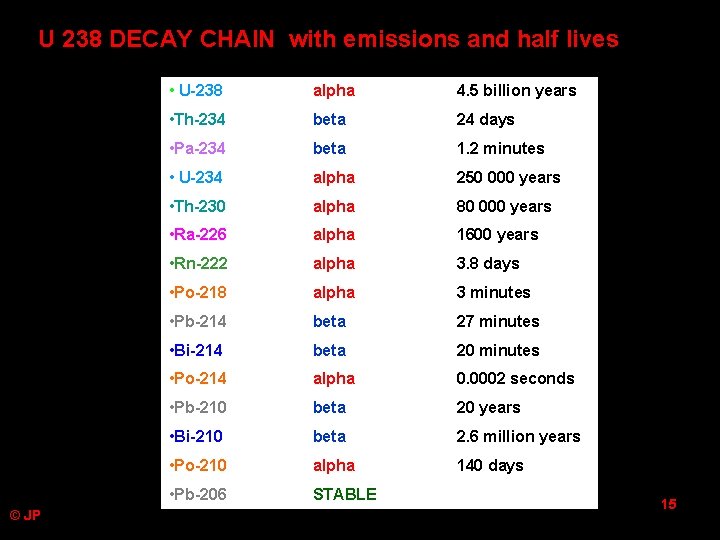
U 238 DECAY CHAIN with emissions and half lives © JP • U-238 alpha 4. 5 billion years • Th-234 beta 24 days • Pa-234 beta 1. 2 minutes • U-234 alpha 250 000 years • Th-230 alpha 80 000 years • Ra-226 alpha 1600 years • Rn-222 alpha 3. 8 days • Po-218 alpha 3 minutes • Pb-214 beta 27 minutes • Bi-214 beta 20 minutes • Po-214 alpha 0. 0002 seconds • Pb-210 beta 20 years • Bi-210 beta 2. 6 million years • Po-210 alpha 140 days • Pb-206 STABLE 15
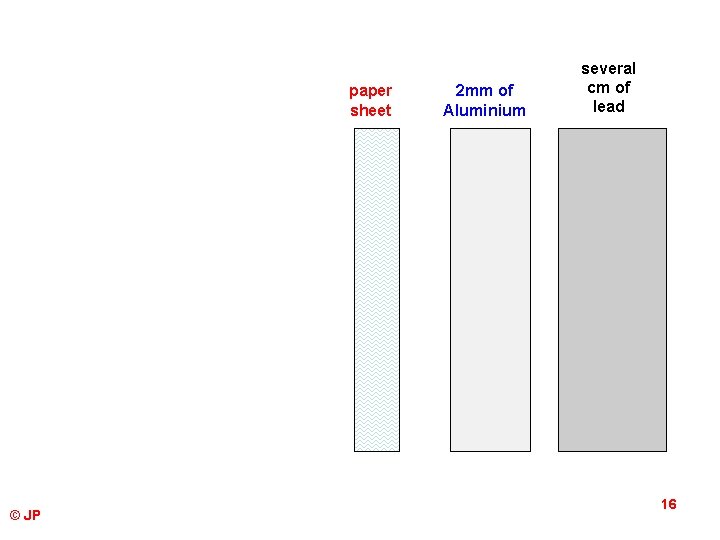
paper sheet © JP 2 mm of Aluminium several cm of lead 16
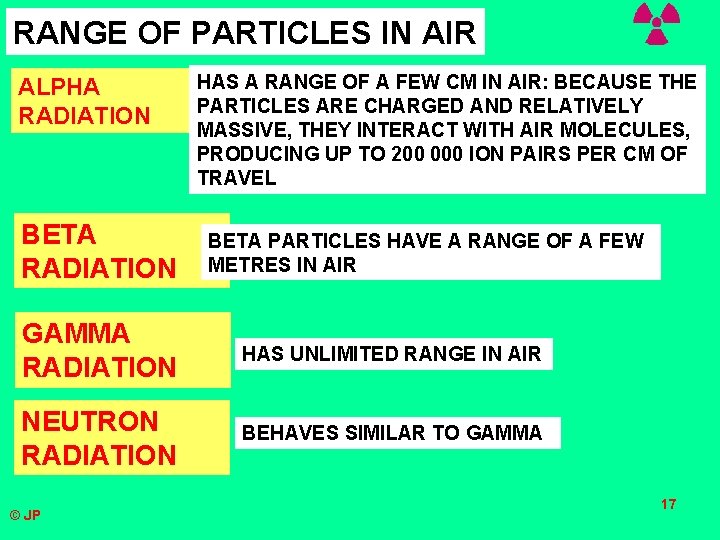
RANGE OF PARTICLES IN AIR ALPHA RADIATION BETA RADIATION HAS A RANGE OF A FEW CM IN AIR: BECAUSE THE PARTICLES ARE CHARGED AND RELATIVELY MASSIVE, THEY INTERACT WITH AIR MOLECULES, PRODUCING UP TO 200 000 ION PAIRS PER CM OF TRAVEL BETA PARTICLES HAVE A RANGE OF A FEW METRES IN AIR GAMMA RADIATION HAS UNLIMITED RANGE IN AIR NEUTRON RADIATION BEHAVES SIMILAR TO GAMMA © JP 17
- Slides: 17