A Bandwidth Detection System to Monitor Single Cells
A Bandwidth Detection System to Monitor Single Cells Jerry J. Wilmink, Jonathon D. Wells, Advisor: Franz Baudenbacher Department of Biomedical Engineering Vanderbilt University BME 273, Group 9, 2002
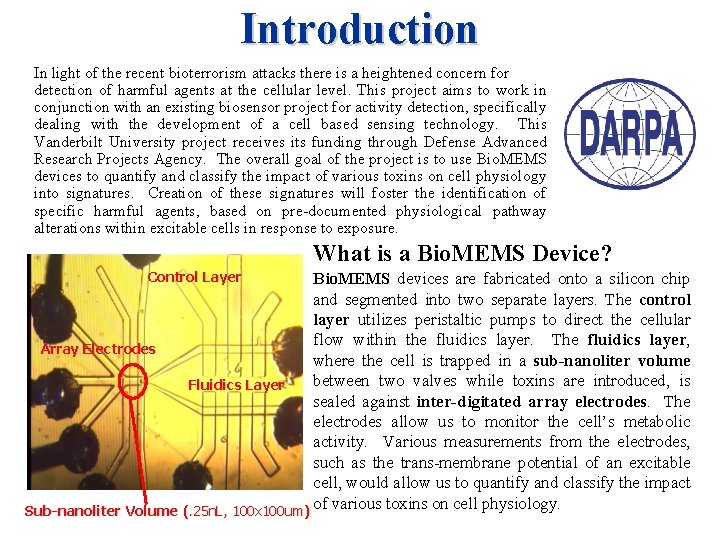
Introduction In light of the recent bioterrorism attacks there is a heightened concern for detection of harmful agents at the cellular level. This project aims to work in conjunction with an existing biosensor project for activity detection, specifically dealing with the development of a cell based sensing technology. This Vanderbilt University project receives its funding through Defense Advanced Research Projects Agency. The overall goal of the project is to use Bio. MEMS devices to quantify and classify the impact of various toxins on cell physiology into signatures. Creation of these signatures will foster the identification of specific harmful agents, based on pre-documented physiological pathway alterations within excitable cells in response to exposure. What is a Bio. MEMS Device? Control Layer Bio. MEMS devices are fabricated onto a silicon chip and segmented into two separate layers. The control layer utilizes peristaltic pumps to direct the cellular flow within the fluidics layer. The fluidics layer, Array Electrodes where the cell is trapped in a sub-nanoliter volume between two valves while toxins are introduced, is Fluidics Layer sealed against inter-digitated array electrodes. The electrodes allow us to monitor the cell’s metabolic activity. Various measurements from the electrodes, such as the trans-membrane potential of an excitable cell, would allow us to quantify and classify the impact Sub-nanoliter Volume (. 25 n. L, 100 x 100 um) of various toxins on cell physiology.

Design Goals ØCreate a design schematic within Innovation Workbench to assist in defining our problem ØDesign the hardware for 4 data acquisition boards, each having 64 channels (256), to read out the Hamamatsu photodiode array incorporating custom designed amplifiers and National Instruments data acquisition boards ØDesign a Labview program to read and record voltages from the 4 x 64 channel NI data acquisition system, to read out a high speed photodiode array, and control the optical imaging recording camera and potentiostat readout from the interdigitiated electrode array ØInterface the photodiode array with an inverted microscope ØDevise a calibration protocol for the manipulation of data acquired from the photodiode array ØRecord the trans-membrane potential of an excitable cell Envisioned Deliverable Design a detection system to measure and visually monitor the transmembrane potential of an excitable cell

Light from Microscope Project Bio. MEMS Device Inverting Microscope Light Voltage Photodiode Array Voltage

Design 2 D Array Output of Intensity of Signal Gain Computer Hardware Amplifier Board NI DAQ Board Data Processing Labview Program

Experimental Bio. MEMS Device Inverting Microscope Photodiode Array

Setup Labview Program Oscilloscope Photodiode NI DAQ Board Power Supply Amplifier Board

Methodology
Step 1: Research and Define Problem Research Problem: Ø Ø DARPA proposal General Bio. MEMS literature Define Problem: Used Innovation Workbench and Design. Safe to define the problem and to address the specific aspects of our design problem Ø Generated a list of equipment needed: Ø • Labview; used to control the 256 -channel data acquisition system, read out a high-speed photodiode array, optical image-recording camera, and potentiostat readout of the interdigitated electrode array • Hamamatsu photodiode array; to generate an appropriate wavelength and to convert to corresponding voltage • National Instruments data acquisition system; used to read out the high speed photodiode array • Inverted Microscope; to observe and document dyes characteristics, corresponding to discrete wavelengths, within a particular excitable cell • Optical Image recording camera • Bio. MEMS devices; to monitor the excitability of cardiac myocytes, neurons, and endothelial cells; excitable cells
Step 2: Equipment Setup and Initial Testing Equipment Setup: Hardware Setup Ø National Instruments 64 -channel Data Acquisition Card inserted into computer port Ø Power Supply manually prepared for connection to Amplifier Board, to provide +/-15 V and +/-7. 5 V Software Setup Ø Installed National Instruments Driver Program Ø Installed NI Labview 6. 0 Initial Testing: Each of the 64 channels on DAQ Board were tested for voltage sensitivity through the Lab. View Testing Panel ROADBLOCK #1: Channel Input Error- Lab. View was reading ½ of our 64 channels through the Test Panel. Resulted from a default channel configuration setting of Differential Readout, thus resulting in the difference between one voltage is referenced to another voltage of a different channel, thus limiting the channels available to 32. Solution: Channels were reconfigured to Sequential Readouts for voltages, for which each channel voltage is referenced to ground.

Step 3: Design Labview VI program Program Capabilities: Ø Designate specific channels to read and store input voltages in a 2 D array Ø Display real-time voltages from specified channels and save data for these selected channels in spreadsheet form Ø Graphically represent input voltage readings while maintaining signal characteristics ROADBLOCK #2: Channel Voltage Drifting- Upon testing random channels using the DAQ Channel Test Panel, neighboring channels were found to be registering errant voltages. Solution: Voltage Drift is a common source of error when neighboring channels on a DAQ panel, which have no input voltage, are not grounded.

Step 4: Modify Lab. View program Here we want to incorporate a visual representation of the position-dependant voltages received from the photodiode array New Program Considerations: Ø The input voltages are stored as a 2 D array, with the column dimension specifying the channel number and the row dimension specifying the Scan Number ROADBLOCK #3: Random Photodiode Pin Layout- There was no simple scheme to organize the array pixels to create a visual representation within Labview, because of the unsystematic layout of pins within the photodiode. Solution: Manually separate each channel by indexing, determine the exact layout of the channels within the photodiode array, and position the individual pixels on the Front Panel according to the position within the photodiode. Ø This array was transposed and indexed to allow data to be extracted from specific channels and repositioned on the front panel of Lab. View Ø Channel layout within the PDA was determined by the pin configuration, with each pin corresponding to a specific channel within Labview Ø Using the position of each channel within the PDA, the coordinates of the color boxes (individual pixels each specific to 1 of 4 DAQ boards, labeled ‘device’ and 1 of 64 channels) were used for positioning within the 16 x 16 array
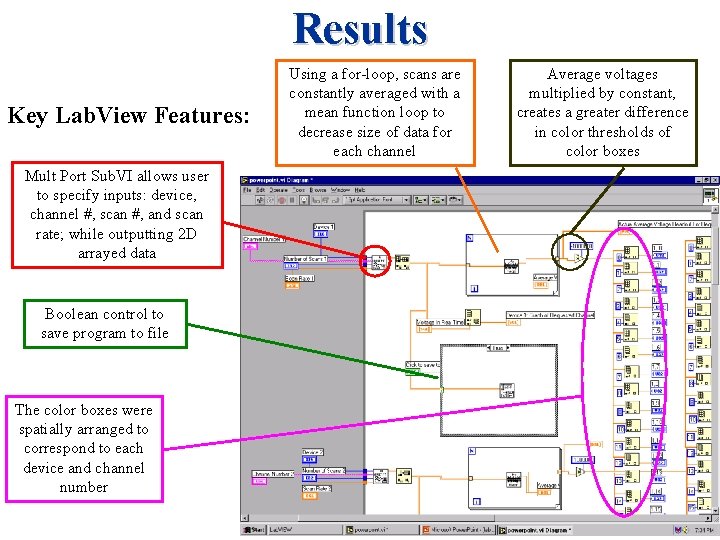
Results Key Lab. View Features: Mult Port Sub. VI allows user to specify inputs: device, channel #, scan #, and scan rate; while outputting 2 D arrayed data Boolean control to save program to file The color boxes were spatially arranged to correspond to each device and channel number Using a for-loop, scans are constantly averaged with a mean function loop to decrease size of data for each channel Average voltages multiplied by constant, creates a greater difference in color thresholds of color boxes
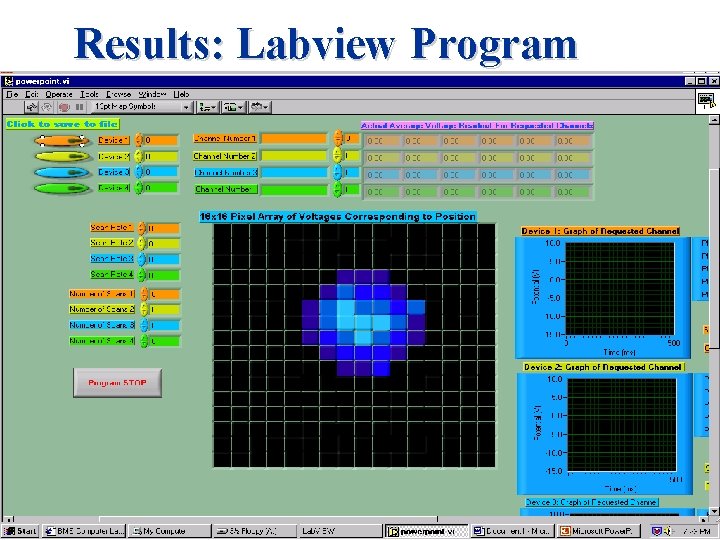
Results: Labview Program

Conclusion A Labview program has been designed to: Ø Read and record voltages from a photodiode array, for a single 64 Channel NI DAQ board Ø Save acquired data into a spreadsheet for future analysis Ø Can graphically represent the analog signal preserving integral signal characteristics for each of the 64 channels Ø 16 X 16 pixel array that represents the intensity of a channel’s voltage as a color

Future Plans Ø The installation of the remaining 3 NI DAQ boards, will allow us to read from all 256 channels, thus completing our 16 X 16 color box array Ø Interface the photodiode array with an inverted microscope and a Bio. MEMS device, thus allowing acquisition of cellular measurements by quantifying discrete wavelengths as voltages, which will be sent to the Labview program for data manipulation Ø Use data to devise a calibration protocol to easily correlate the voltages from the 256 channels to the trans-membrane potential of an excitable cell Ø Record the trans-membrane potential of excitable cells, and begin creating signatures to quantify the quantify and classify the impact of various toxins on cell physiology Acknowledgements This work was completed using the funding provided by the DARPA grant (1) Andreas References (2) Roger Hebert, Sales Rep. for National Instruments (3) National Instruments Lab. View 6. 0 Instruction Manual and Measurements and Analysis Manual
- Slides: 16