a 3 Dimensional Microarray Substrate Bio Chip Ventures

a 3 -Dimensional Microarray Substrate Bio. Chip Ventures Division
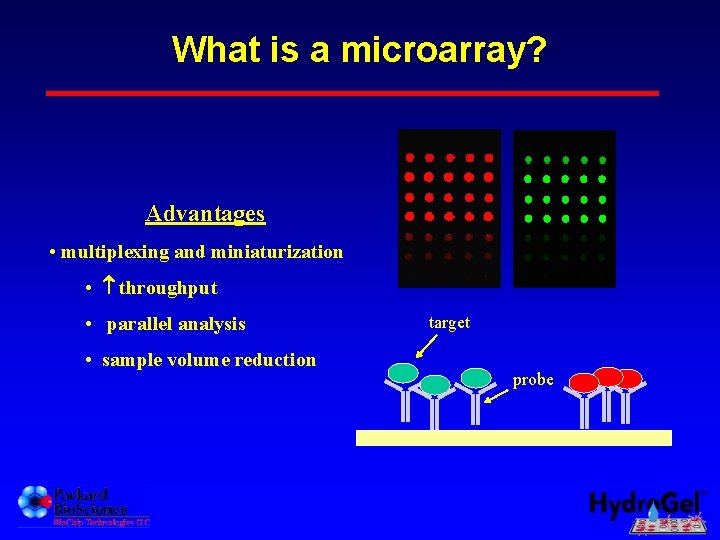
What is a microarray? Advantages • multiplexing and miniaturization • throughput • parallel analysis • sample volume reduction target probe
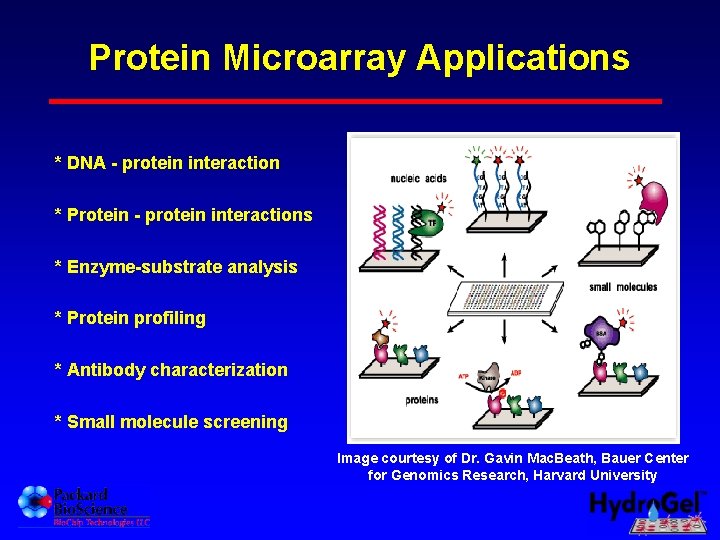
Protein Microarray Applications * DNA - protein interaction * Protein - protein interactions * Enzyme-substrate analysis * Protein profiling * Antibody characterization * Small molecule screening Image courtesy of Dr. Gavin Mac. Beath, Bauer Center for Genomics Research, Harvard University
Desirable Substrate Properties for Protein Microarray Applications • Protein compatible • High probe loading capacity • Low inherent fluorescence and nonspecific binding background • Consistent, uniform product • Ease of use

Hydro. Gel. TM Coated Slides
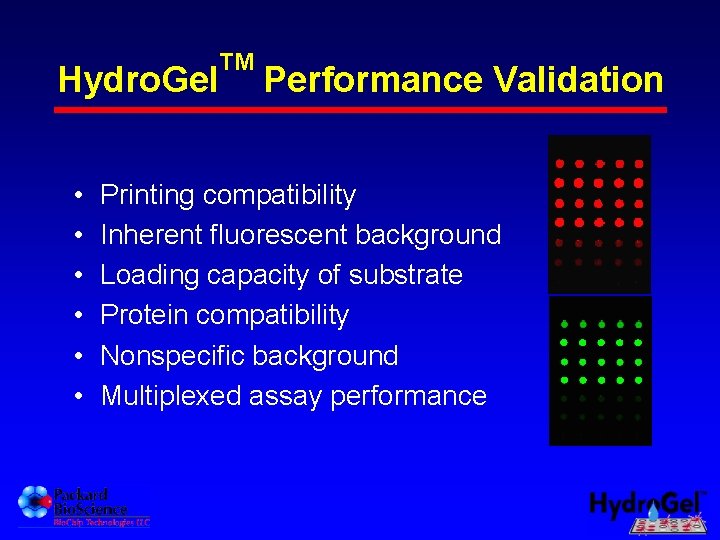
Hydro. Gel • • • TM Performance Validation Printing compatibility Inherent fluorescent background Loading capacity of substrate Protein compatibility Nonspecific background Multiplexed assay performance

Printing Compatibility Packard Bio. Chip Arrayer. TM (Piezo) Feature Size ~200 um Packard Spot. Array 24 (Split Pin) Feature Size ~150 um Hydro. Gel. TM coated slides are compatible with both contact and non-contact printers (examples shown above). This was verified on two other types of commercially available and “home-made” instruments.
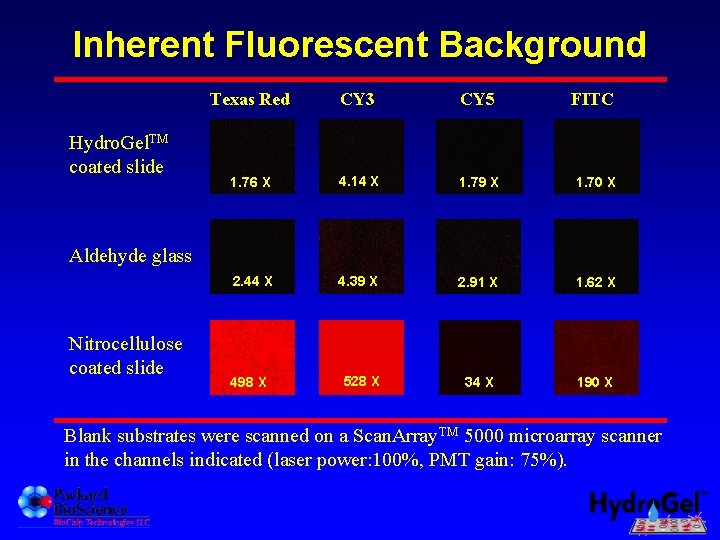
Inherent Fluorescent Background Hydro. Gel. TM coated slide Texas Red CY 3 CY 5 FITC 1. 76 X 4. 14 X 1. 79 X 1. 70 X 2. 44 X 4. 39 X 2. 91 X 1. 62 X 498 X 528 X 34 X 190 X Aldehyde glass Nitrocellulose coated slide Blank substrates were scanned on a Scan. Array. TM 5000 microarray scanner in the channels indicated (laser power: 100%, PMT gain: 75%).
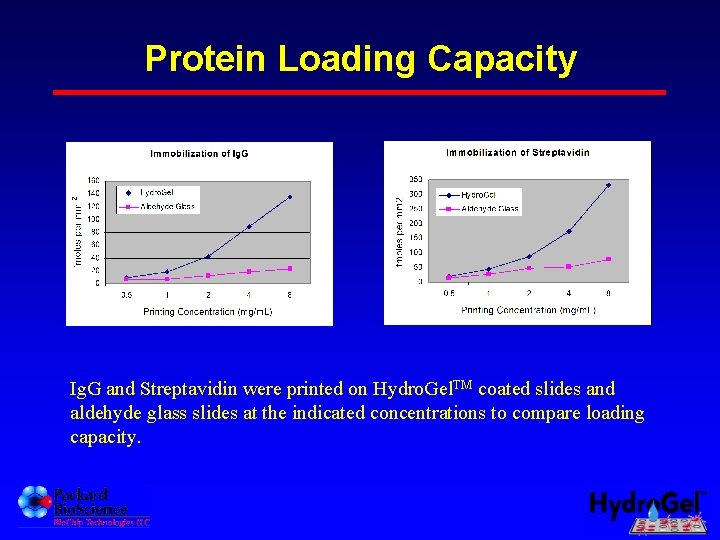
Protein Loading Capacity Ig. G and Streptavidin were printed on Hydro. Gel. TM coated slides and aldehyde glass slides at the indicated concentrations to compare loading capacity.
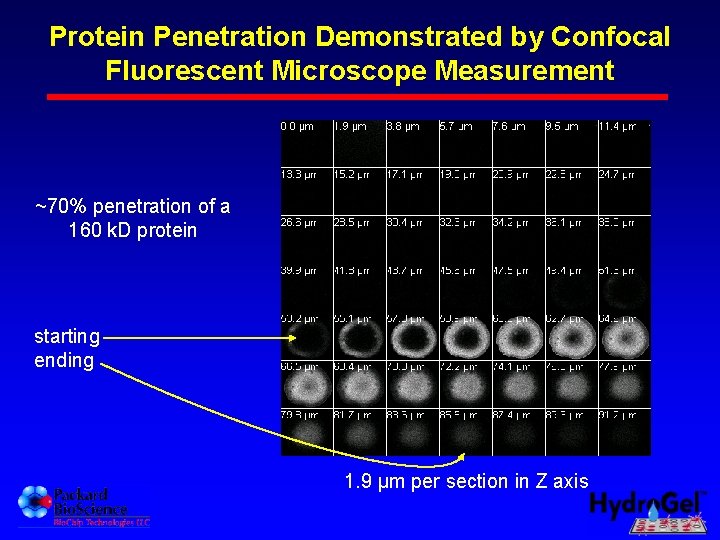
Protein Penetration Demonstrated by Confocal Fluorescent Microscope Measurement ~70% penetration of a 160 k. D protein starting ending 1. 9 µm per section in Z axis
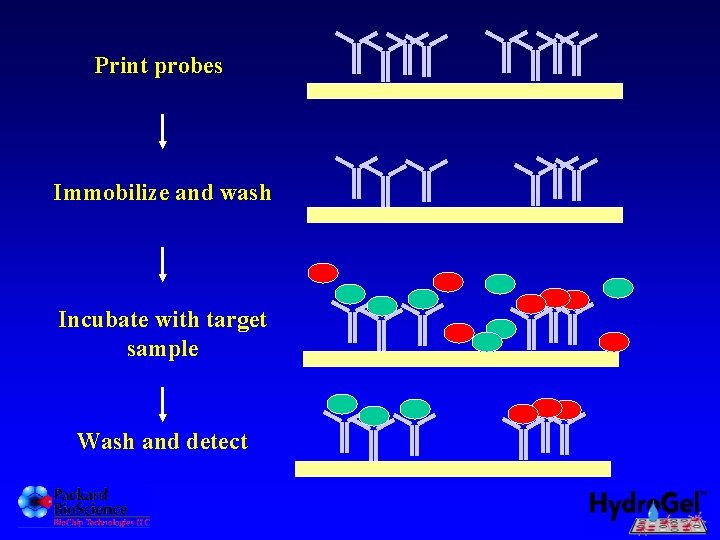
Print probes Immobilize and wash Incubate with target sample Wash and detect
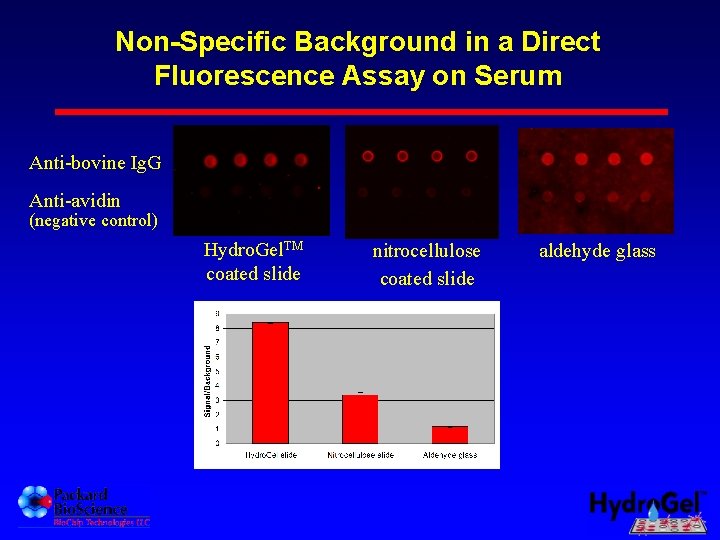
Non-Specific Background in a Direct Fluorescence Assay on Serum Anti-bovine Ig. G Anti-avidin (negative control) Hydro. Gel. TM coated slide nitrocellulose coated slide aldehyde glass
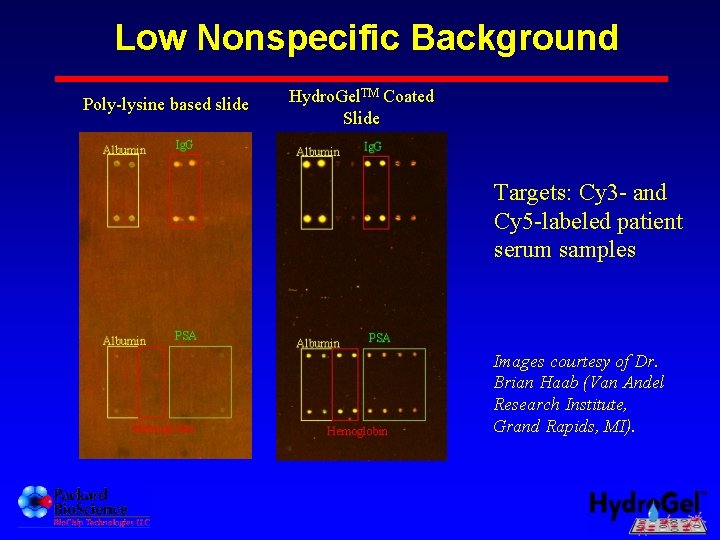
Low Nonspecific Background Poly-lysine based slide Hydro. Gel. TM Coated Slide Targets: Cy 3 - and Cy 5 -labeled patient serum samples Images courtesy of Dr. Brian Haab (Van Andel Research Institute, Grand Rapids, MI).
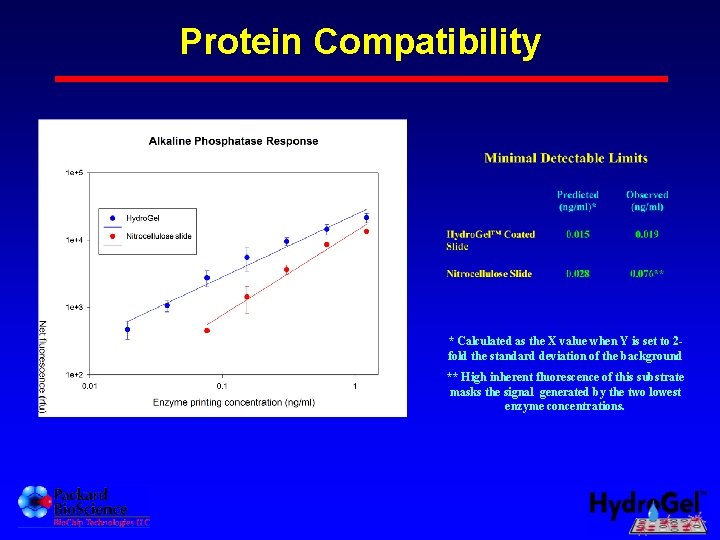
Protein Compatibility * Calculated as the X value when Y is set to 2 fold the standard deviation of the background ** High inherent fluorescence of this substrate masks the signal generated by the two lowest enzyme concentrations.
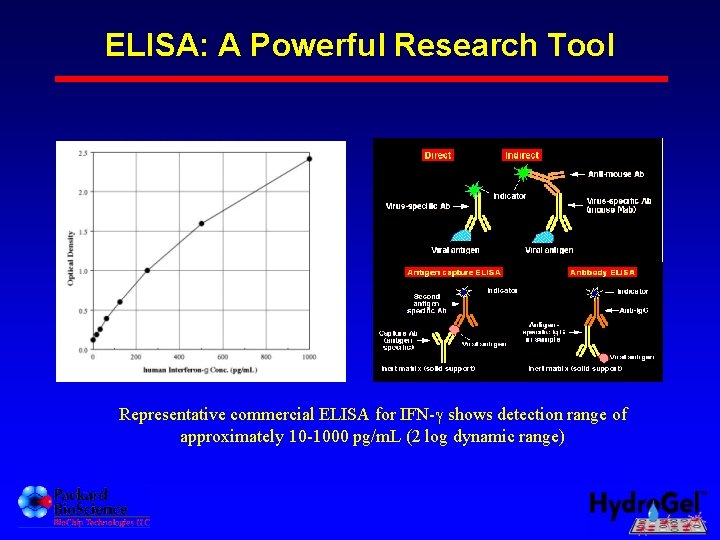
ELISA: A Powerful Research Tool Representative commercial ELISA for IFN- shows detection range of approximately 10 -1000 pg/m. L (2 log dynamic range)
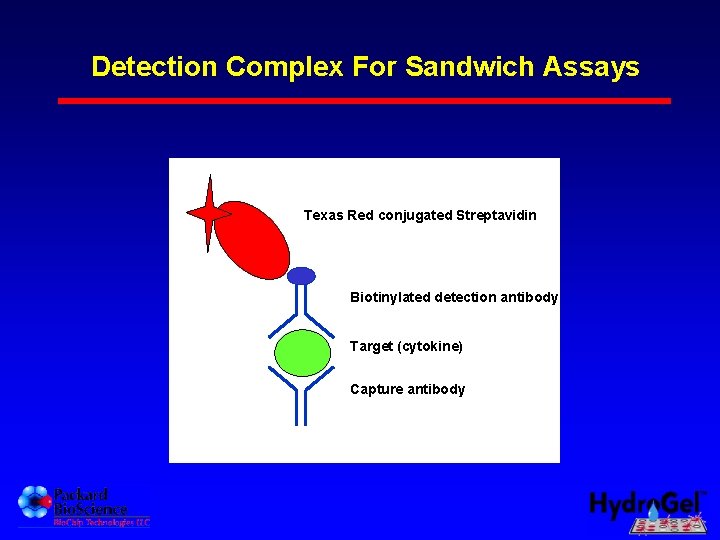
Detection Complex For Sandwich Assays Texas Red conjugated Streptavidin Biotinylated detection antibody Target (cytokine) Capture antibody
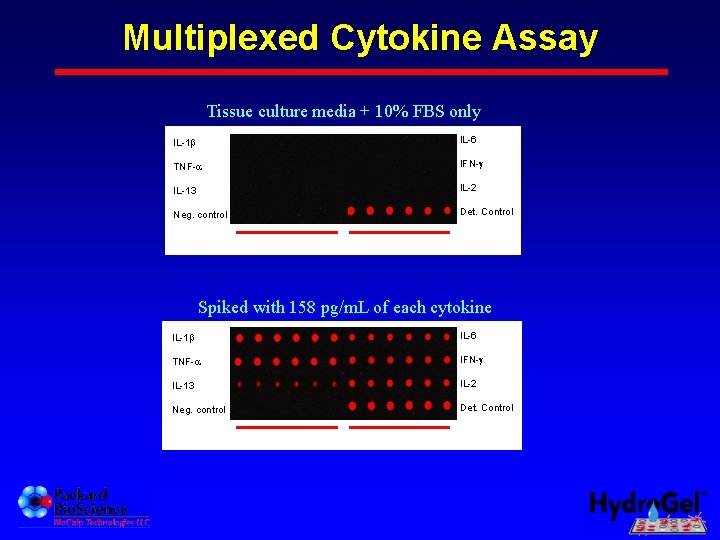
Multiplexed Cytokine Assay Tissue culture media + 10% FBS only IL-1 IL-6 TNF- IFN- IL-13 IL-2 Neg. control Det. Control replicates Spiked with 158 pg/m. L of each cytokine IL-1 IL-6 TNF- IFN- IL-13 IL-2 Neg. control Det. Control replicates
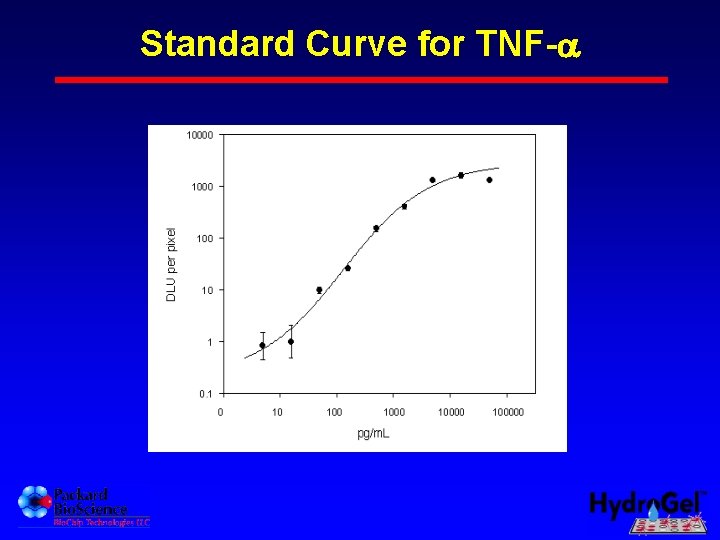
Standard Curve for TNF-a TNF-
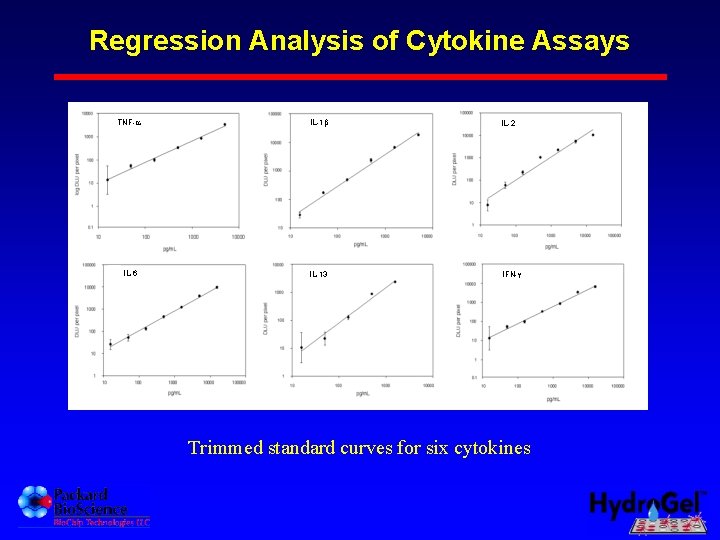
Regression Analysis of Cytokine Assays B. TNF- C. IL-1 D. IL-2 E. IL-6 F. IL-13 G. IFN- Trimmed standard curves for six cytokines
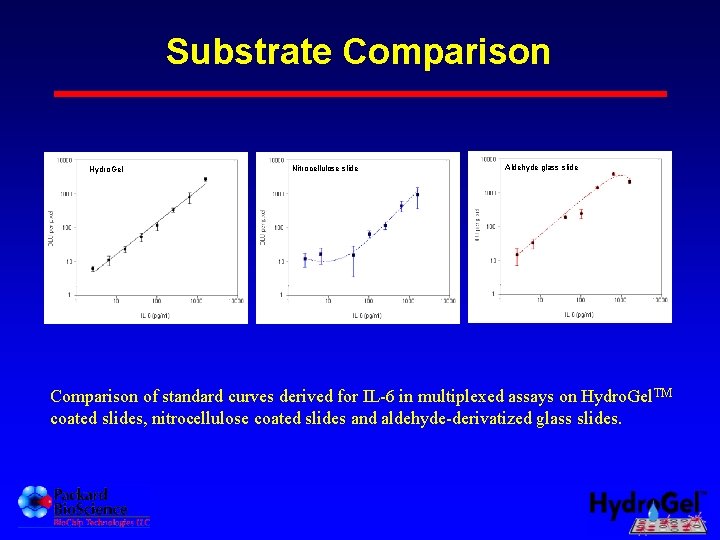
Substrate Comparison Hydro. Gel A. Nitrocellulose slide Nitrocellulose B. Aldehyde glass slide C. Comparison of standard curves derived for IL-6 in multiplexed assays on Hydro. Gel. TM coated slides, nitrocellulose coated slides and aldehyde-derivatized glass slides.
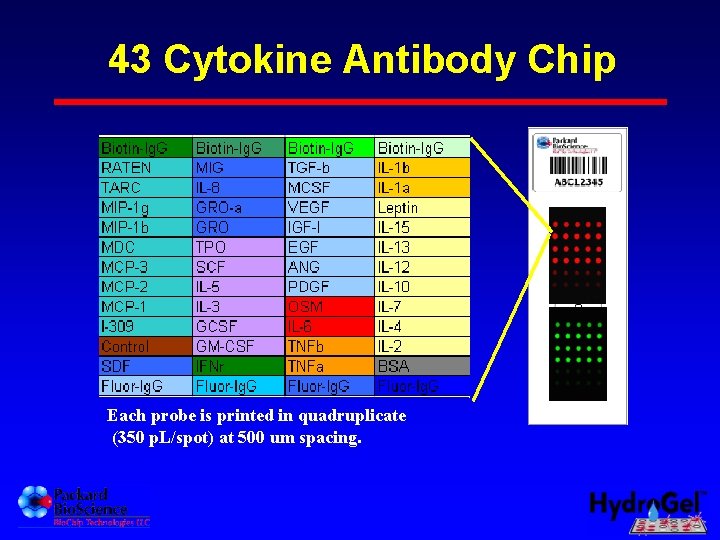
43 Cytokine Antibody Chip Each probe is printed in quadruplicate (350 p. L/spot) at 500 um spacing.
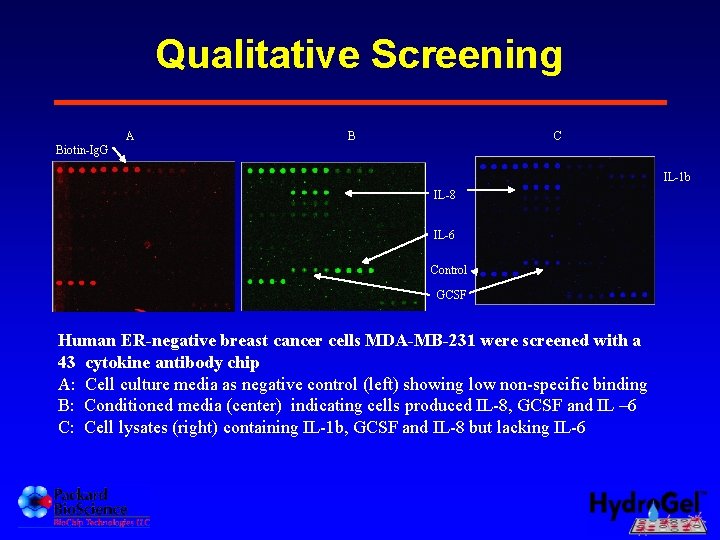
Qualitative Screening A B C Biotin-Ig. G IL-1 b IL-8 IL-6 Control GCSF Human ER-negative breast cancer cells MDA-MB-231 were screened with a 43 cytokine antibody chip A: Cell culture media as negative control (left) showing low non-specific binding B: Conditioned media (center) indicating cells produced IL-8, GCSF and IL – 6 C: Cell lysates (right) containing IL-1 b, GCSF and IL-8 but lacking IL-6
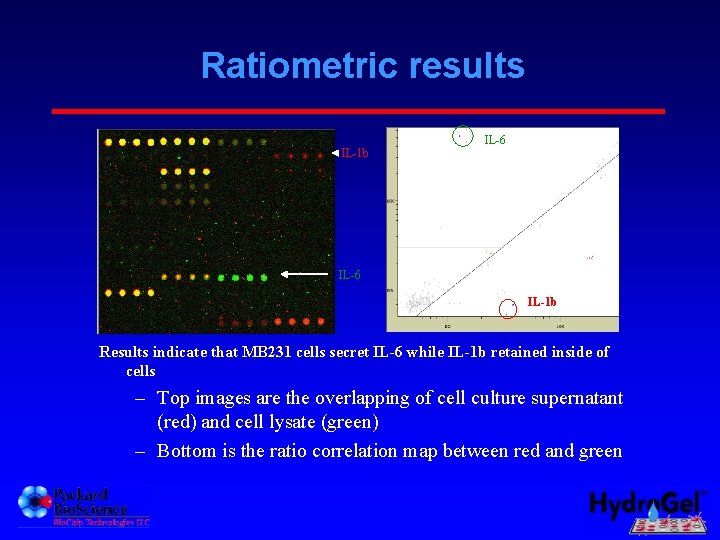
Ratiometric results IL-1 b IL-6 IL-1 b Results indicate that MB 231 cells secret IL-6 while IL-1 b retained inside of cells – Top images are the overlapping of cell culture supernatant (red) and cell lysate (green) – Bottom is the ratio correlation map between red and green
Summary • compatible with both contact and non-contact printing • low inherent fluorescence and nonspecific background • higher functional protein loading capacity • 3 -dimensional, hydrophilic environment seems to maintain protein structure and promotes functionality • superior assay performance • high sensitivity • broad dynamic range
- Slides: 24