95 THE MYSTERY OF THE BLUE PRECIPITATE OBJECTIVE

9/5: THE MYSTERY OF THE BLUE PRECIPITATE OBJECTIVE: 1. IDENTIFY AND DESCRIBE SCIENCE METHODS. TASK: PLEASE COPY: Hypothesis: A possible explanation or solution. Prediction: An if / then statement that describe what is expected if a hypothesis is true. Data: measurements & observations PLEASE COPY:
SHARE OUT: 1. Work with your 1 -2 neighbors to make a combined list. 2. We will then share out by group. If another group uses one of your responses, cross it off your list

SCIENCE METHODS TO IDENTIFY THE BLUE SOLID… • 1: Questioning: What is that unexpected blue solid? • 2: Investigation: • My first working hypothesis was that the precipitate was the result of a chemical reaction between oxygen and metals like Calcium or Magnesium that are found in “hard” water. • I did an experiment to test my hypothesis. This was my prediction: if the oxygen is reacting with minerals in hard water, then the reaction will stop when the minerals are used up • . I filtered the water, then tried the experiment again: if the precipitate was from minerals in the water, they should be used up and no blue precipitate should form the 2 nd try. • 3: Analyze & Interpret Data: MY FIRST HYPOTHESIS WAS WRONG! Because the blue solid kept being formed, I knew it must be something other than minerals in the water! • 4: Use information: If the source of metal wasn’t the tap water, it must be copper or nickel, from the wires. I used Google and Wikipedia to research different compounds that contain nickel or copper and oxygen. I found the picture shown on the Wikipedia entry for “Copper II Hydroxide”

SCIENCE METHODS TO IDENTIFY THE BLUE SOLID… • 4: Use Information (again): A few pieces of information from Wikipedia supported my new hypothesis that the blue stuff is copper II hydroxide: • “It is a pale greenish blue or bluish green solid. ” • “copper hydroxide is readily made by electrolysis of water with a copper anode. ” (The anode is the positive wire) This is where things stand at the moment. So are we done? Is the blue precipitate definitely copper hydroxide?

NO WAY! SCIENCE DEMANDS DATA & OBSERVATION • My new hypothesis (“The blue stuff is copper hydroxide”) needs to be tested! • Predictions test hypotheses: • If the blue stuff is copper hydroxide then _____________ • Hint 1: “copper hydroxide is readily made by electrolysis of water with a copper anode. ” • Hint 2: “[copper hydroxide] slowly reacts with carbon dioxide from the atmosphere to form a basic copper(II) carbonate. Thus copper slowly acquires a dull green coating in moist air… This… forms on bronze and other copper alloy statues such as the Statue of Liberty.

DID THE BLUE STUFF TURN GREEN OVERNIGHT? • I made this powerpoint before observing the sample today, but: • IF THE UNKNOWN SUBSTANCE IS COPPER HYDROXIDE THEN IT MAY HAVE TURNED A LITTLE GREEN OVERNIGHT • So: What color is it? Does this support or refute my hypothesis?

TIME TO EXPERIMENT! START A JOURNAL ENTRY: Identifying the Mysterious Blue Solid (9/5) Write: HYPOTHESIS: The mysterious blue solid is copper II hydroxide. Write: PREDICTION: If the solid is copper hydroxide [Cu(OH)2] then… Uh oh: what is your experiment going to be? What can you change about the design of the experiment that will make it NOT produce copper hydroxide if we’re right?
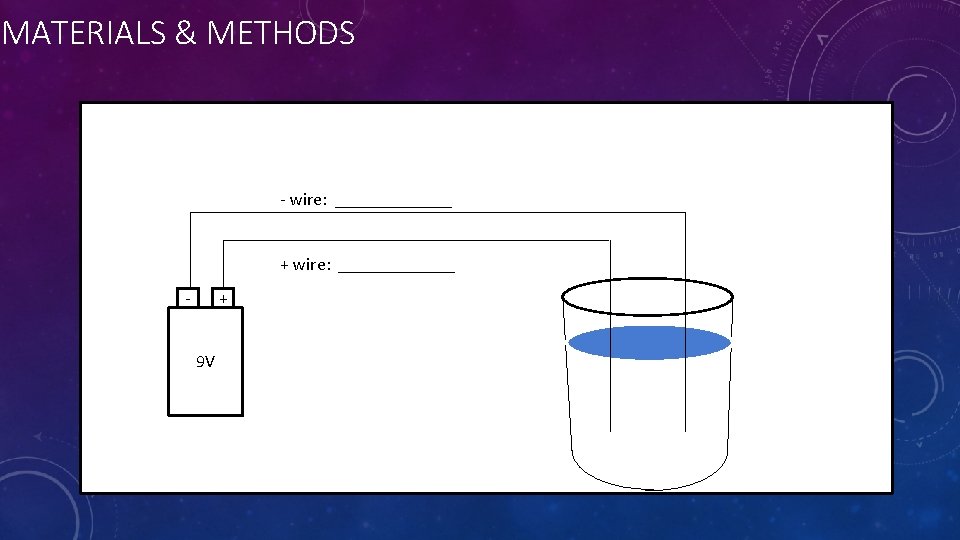
MATERIALS & METHODS - wire: _______ + wire: _______ - + 9 V
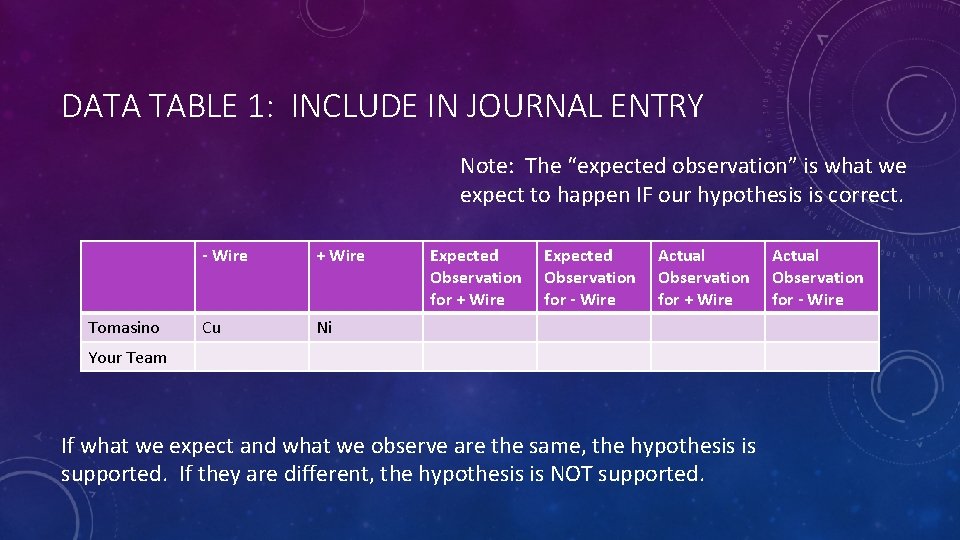
DATA TABLE 1: INCLUDE IN JOURNAL ENTRY Note: The “expected observation” is what we expect to happen IF our hypothesis is correct. Tomasino - Wire + Wire Cu Ni Expected Observation for + Wire Expected Observation for - Wire Actual Observation for + Wire Your Team If what we expect and what we observe are the same, the hypothesis is supported. If they are different, the hypothesis is NOT supported. Actual Observation for - Wire

NOW GO BUILD IT AND TEST! BUT First: Which teams are testing each combination of wires? • To build the apparatus: • • CHECK TO MAKE SURE YOUR DIAGRAM, DATA TABLE, and APPARATUS ALL HAVE THE SAME ANODE AND CATHODE MATERIALS. Wrap the exposed metal on one end around the battery terminal to make good contact. Tape in place with electrical tape. Do the same for the other wire and terminal. • NOTE: Each wire must touch only 1 battery terminal. • NOTE: Do not allow the two free ends of the wires to touch: we need the electricity to travel THROUGH the water. • NOTE: Make sure you record which materials your anode and cathode wires are in your data table. Tape the wires to the cup of water, and bend them so the exposed metal is under water.
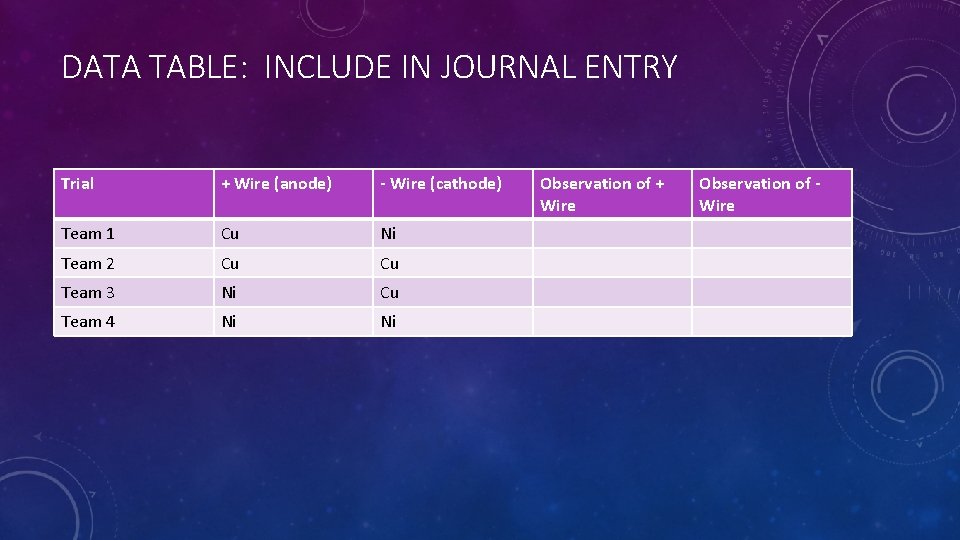
DATA TABLE: INCLUDE IN JOURNAL ENTRY Trial + Wire (anode) - Wire (cathode) Team 1 Cu Ni Team 2 Cu Cu Team 3 Ni Cu Team 4 Ni Ni Observation of + Wire Observation of Wire
- Slides: 11