93013 VALENCE ELECTRONS BOHR MODELS Atom Structure Most

9/30/13 VALENCE ELECTRONS & BOHR MODELS
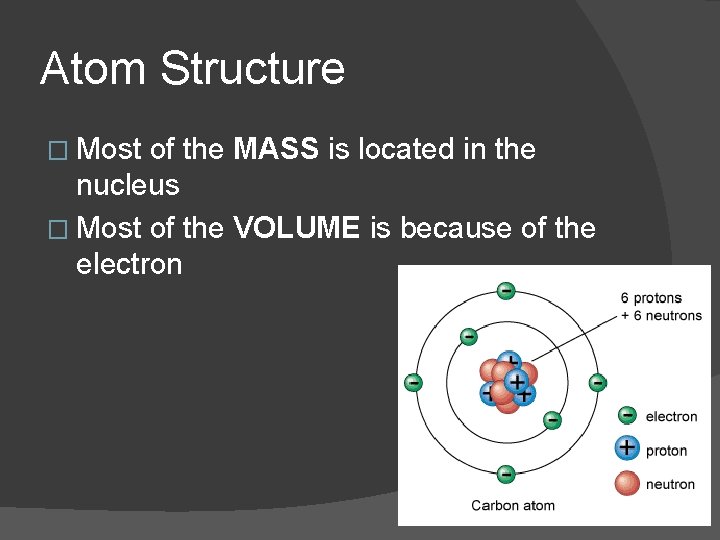
Atom Structure � Most of the MASS is located in the nucleus � Most of the VOLUME is because of the electron
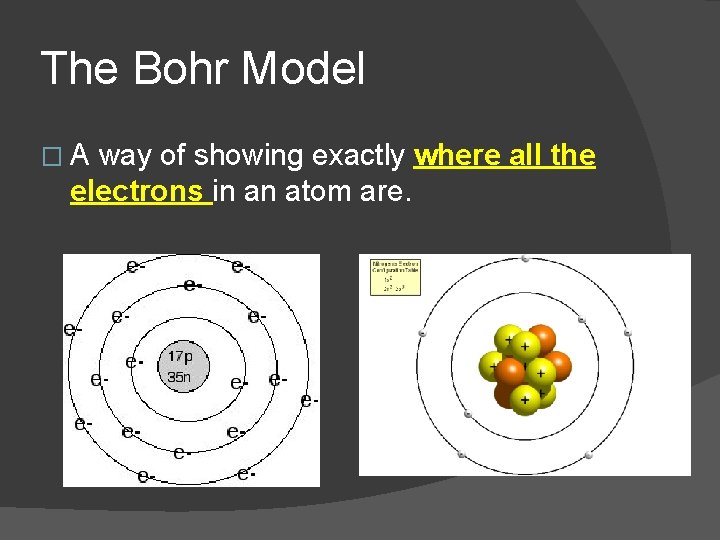
The Bohr Model �A way of showing exactly where all the electrons in an atom are.
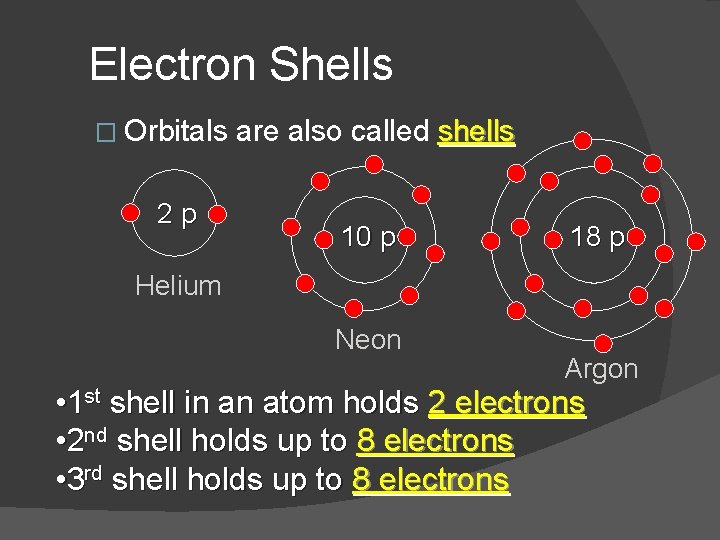
Electron Shells � Orbitals 2 p are also called shells 10 p 18 p Helium Neon Argon • 1 st shell in an atom holds 2 electrons • 2 nd shell holds up to 8 electrons • 3 rd shell holds up to 8 electrons
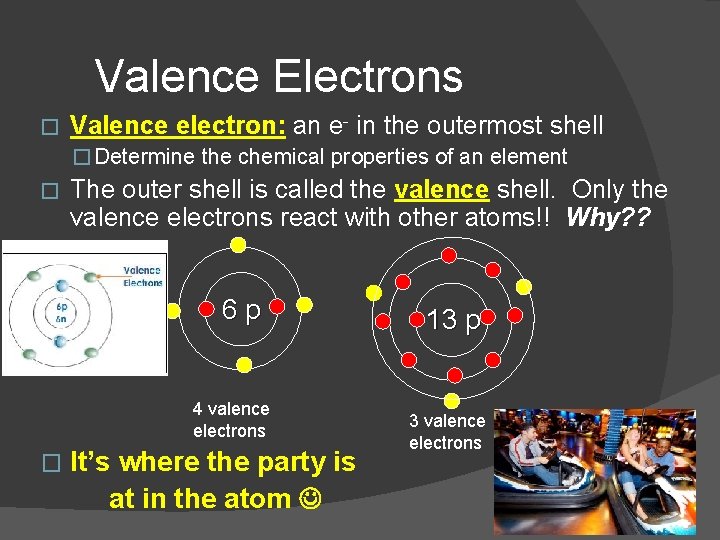
Valence Electrons � Valence electron: an e- in the outermost shell � Determine the chemical properties of an element � The outer shell is called the valence shell. Only the valence electrons react with other atoms!! Why? ? 6 p 4 valence electrons � It’s where the party is at in the atom 13 p 3 valence electrons

Let it out! � Why are valence electrons important for chemical reactions?
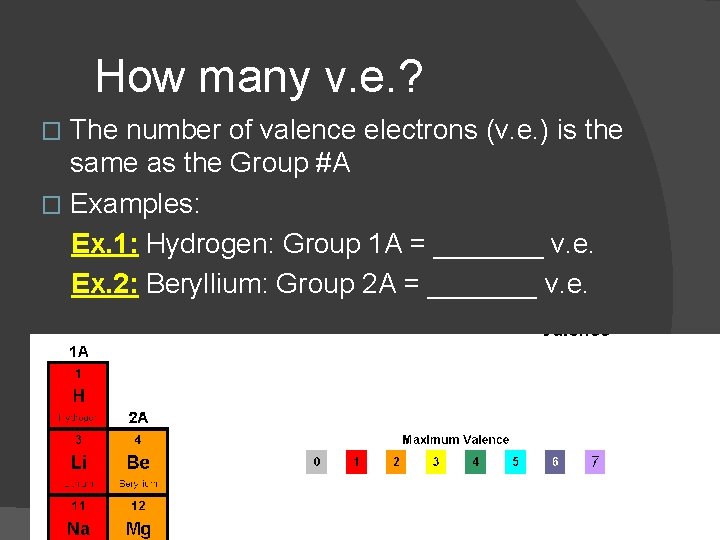
How many v. e. ? The number of valence electrons (v. e. ) is the same as the Group #A � Examples: Ex. 1: Hydrogen: Group 1 A = _______ v. e. Ex. 2: Beryllium: Group 2 A = _______ v. e. �

Practice Element 1. Beryllium 2. Magnesium 3. Sodium 4. Lithium 5. Silicon 6. Fluorine 7. Chlorine Group # # of v. e.

What patterns can we find? ?
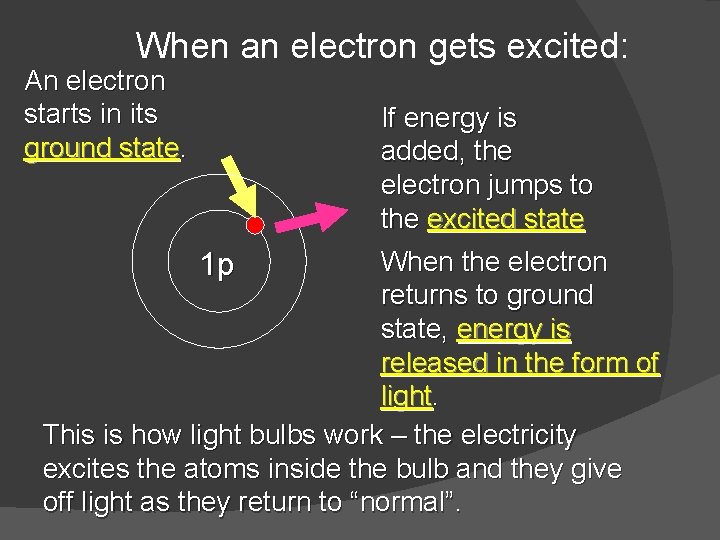
When an electron gets excited: An electron starts in its ground state. If energy is added, the electron jumps to the excited state When the electron returns to ground state, energy is released in the form of light. This is how light bulbs work – the electricity excites the atoms inside the bulb and they give off light as they return to “normal”. 1 p

Excited State Video…
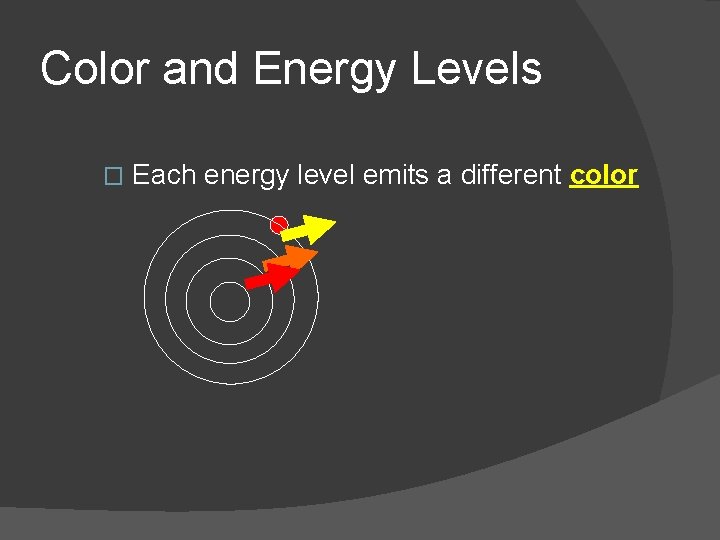
Color and Energy Levels � Each energy level emits a different color

WHERE ELSE HAVE WE SEEN COLORED LIGHT COMING FROM EXCITED ELECTRONS? ? Fireworks!!

Summary � How do you tell the number of valence electrons in an atom?


• Why do grocery stores organize their food?

• How does every grocery store organize their food?
Periodic Table is like a grocery store…
In your groups find 4 patterns in periodic table � You have 3 minutes!
- Slides: 19