9 7 NOTES Decomposition Decomposition reactions These reactions






- Slides: 10

9. 7 – NOTES Decomposition

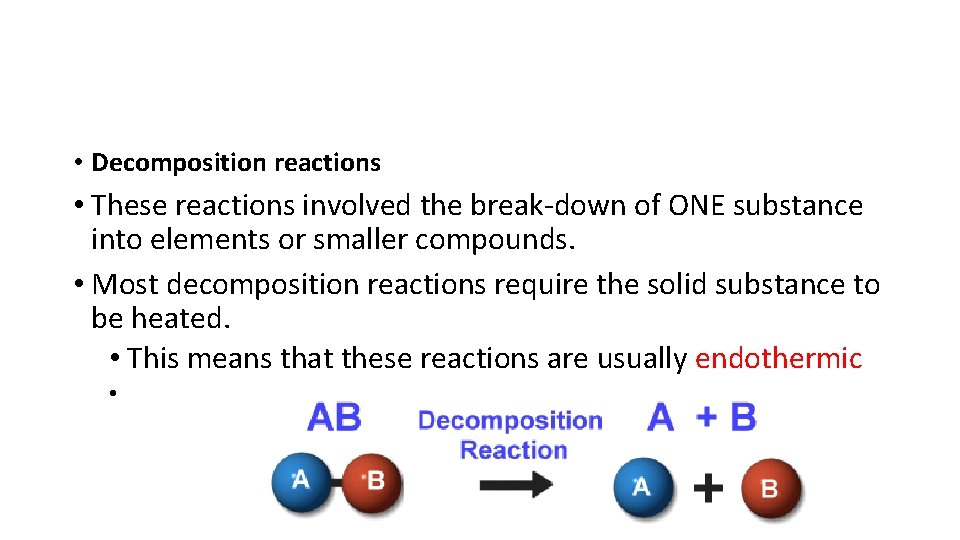
• Decomposition reactions • These reactions involved the break-down of ONE substance into elements or smaller compounds. • Most decomposition reactions require the solid substance to be heated. • This means that these reactions are usually endothermic •


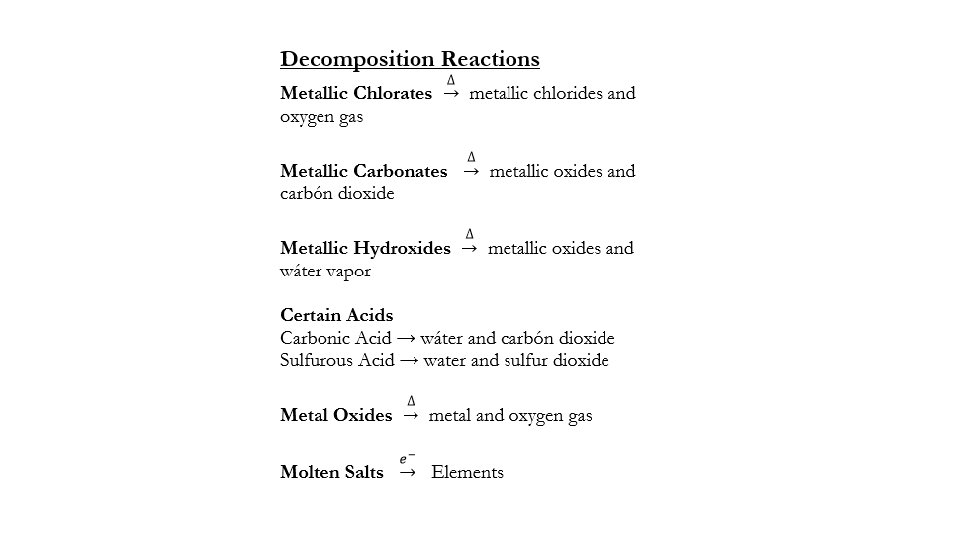
• 1. Metallic chlorates • When heated, these will produce metal chlorides and oxygen gas; • Sodium chlorate is heated. • Ga(Cl. O 3)3 (s) Ga. Cl 3 (s)+ 4. 5 O 2 (g)

• 2. Metallic Carbonates • Whern heated, these will produce a metal oxide and carbon dioxide; • Lead (II) Carbonate is heated Al 2(CO 3)3 (s) Al 2 O 3 (s) + 3 CO 2 (g)

• 3. Metallic hydroxides • When heated, these will produce a metal oxide and water vapor • 2 Li. OH (s) Li 2 O (s) + H 2 O (g) • Ti(OH)4 (s) Ti. O 2 (s) + 2 H 2 O (g)

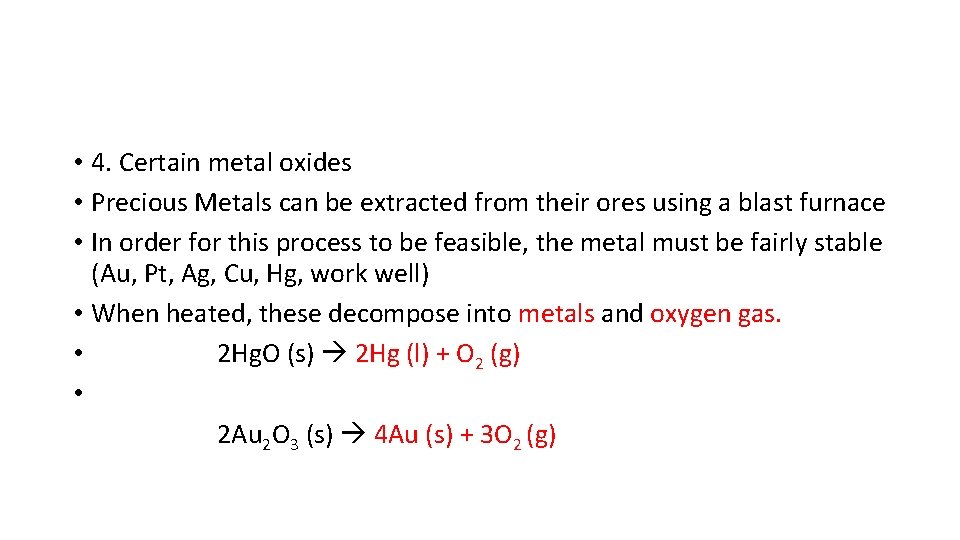
• 4. Certain metal oxides • Precious Metals can be extracted from their ores using a blast furnace • In order for this process to be feasible, the metal must be fairly stable (Au, Pt, Ag, Cu, Hg, work well) • When heated, these decompose into metals and oxygen gas. • 2 Hg. O (s) 2 Hg (l) + O 2 (g) • 2 Au 2 O 3 (s) 4 Au (s) + 3 O 2 (g)

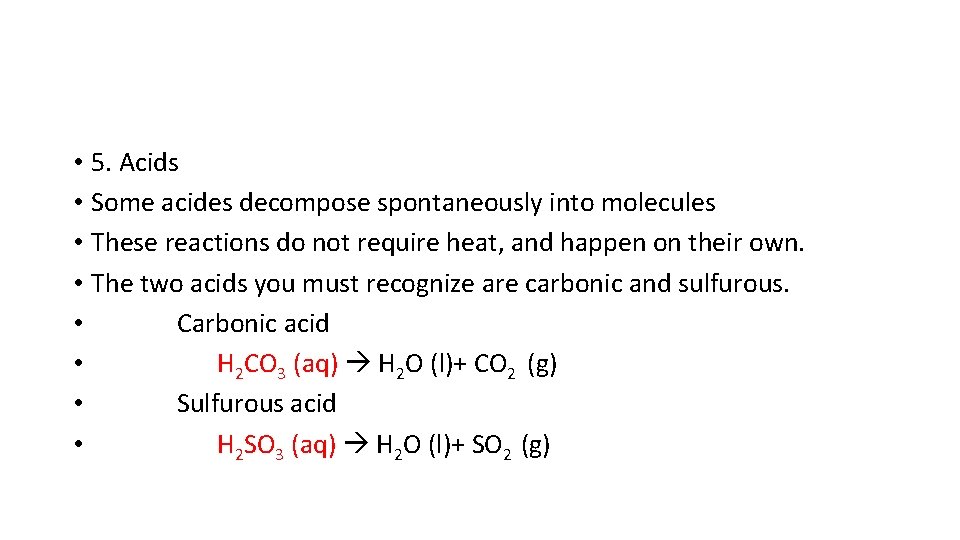
• 5. Acids • Some acides decompose spontaneously into molecules • These reactions do not require heat, and happen on their own. • The two acids you must recognize are carbonic and sulfurous. • Carbonic acid • H 2 CO 3 (aq) H 2 O (l)+ CO 2 (g) • Sulfurous acid • H 2 SO 3 (aq) H 2 O (l)+ SO 2 (g)

• 6. Electrolysis of molten salts • These reactions involve the use of electricity to split a salt (ionic compound) into elements • In order for this type of reaction to occur, the salt must first be in a molten state (liquid) • Then, current is applied to the molten salt so that elements form. • This is the only way that alkali metals can be made into their elemental state. • Molten Li. Br: 2 Li. Br (l) 2 Li (l) + Br 2 (l) Ca. Cl 2 (l) Ca (l) + Cl 2 (g)
