9 5 Acidity of Acetylene and Terminal Alkynes






- Slides: 21

9. 5 Acidity of Acetylene and Terminal Alkynes H C C

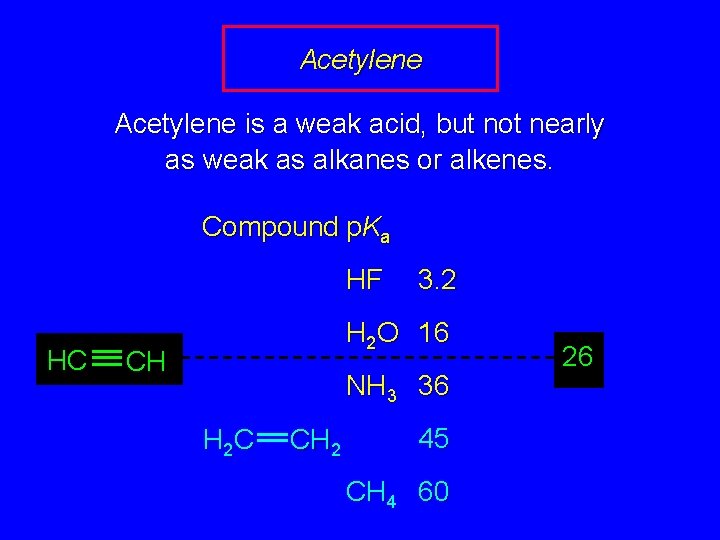
Acidity of Hydrocarbons In general, hydrocarbons are exceedingly weak acids Compound p. Ka HF 3. 2 H 2 O 16 NH 3 36 H 2 C CH 2 45 CH 4 60

Acetylene is a weak acid, but not nearly as weak as alkanes or alkenes. Compound p. Ka HF HC 3. 2 H 2 O 16 CH NH 3 36 H 2 C CH 2 45 CH 4 60 26

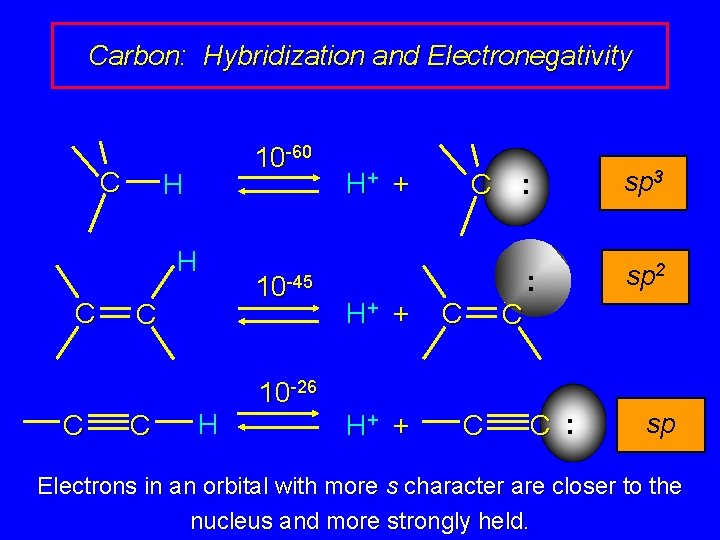
Carbon: Hybridization and Electronegativity C 10 -60 H H C C H 10 -45 H+ + C C 10 -26 H+ + C : sp 3 : sp 2 C C : sp Electrons in an orbital with more s character are closer to the nucleus and more strongly held.

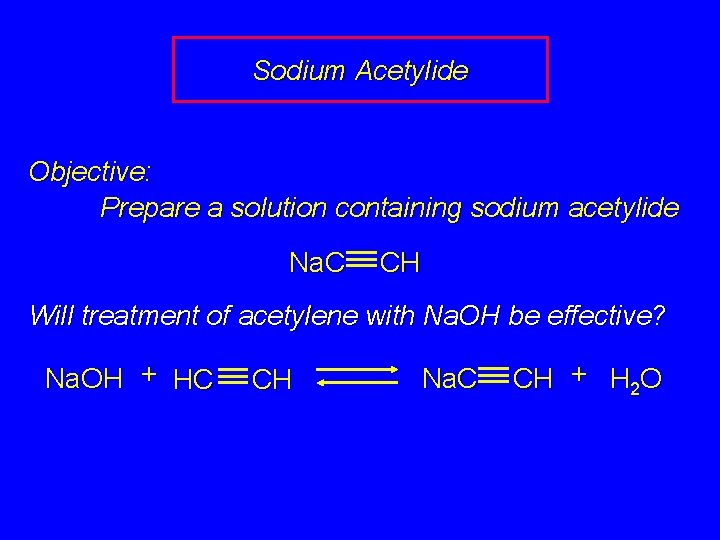
Sodium Acetylide Objective: Prepare a solution containing sodium acetylide Na. C CH Will treatment of acetylene with Na. OH be effective? Na. OH + HC CH Na. C CH + H 2 O

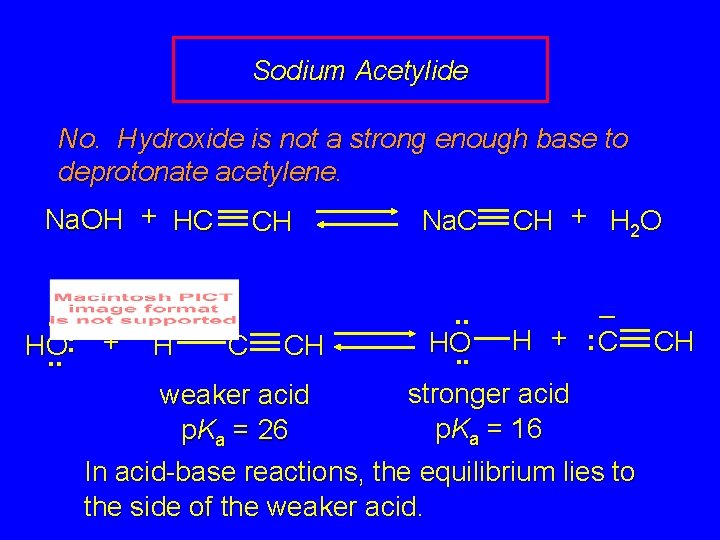
Sodium Acetylide No. Hydroxide is not a strong enough base to deprotonate acetylene. Na. OH + HC. . – : + HO. . H CH C CH Na. C CH + H 2 O . . – + : C H HO. . stronger acid p. Ka = 16 weaker acid p. Ka = 26 In acid-base reactions, the equilibrium lies to the side of the weaker acid. CH

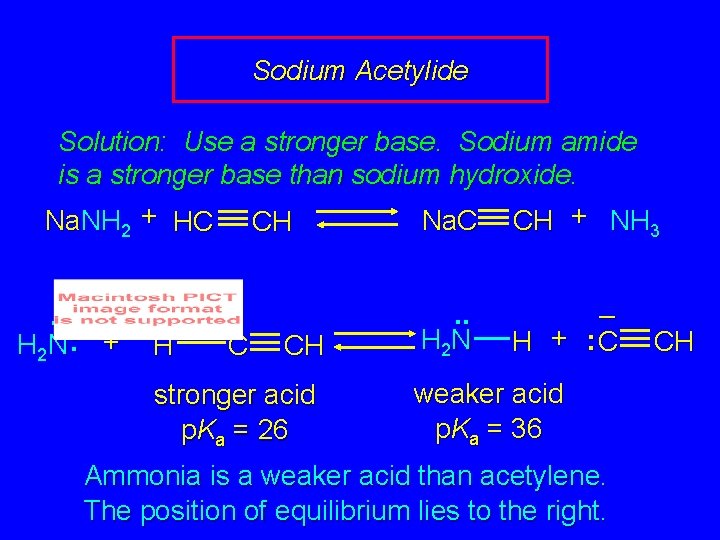
Sodium Acetylide Solution: Use a stronger base. Sodium amide is a stronger base than sodium hydroxide. Na. NH 2 + HC CH Na. C CH + NH 3. . – H 2 N : + H C CH stronger acid p. Ka = 26 . . H 2 N – H + : C weaker acid p. Ka = 36 Ammonia is a weaker acid than acetylene. The position of equilibrium lies to the right. CH

9. 6 Preparation of Alkynes by Alkylation of Acetylene and Terminal Alkynes

Preparation of Alkynes There are two main methods for the preparation of alkynes: Carbon-carbon bond formation alkylation of acetylene and terminal alkynes Functional-group transformations elimination

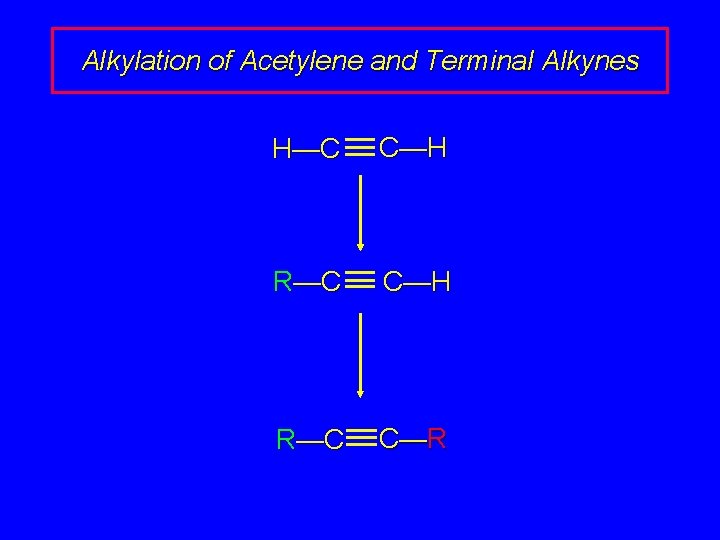
Alkylation of Acetylene and Terminal Alkynes H—C C—H R—C C—R

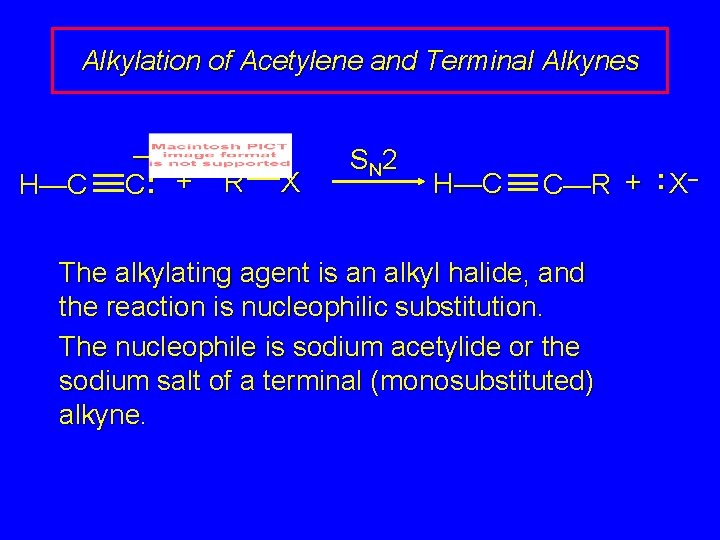
Alkylation of Acetylene and Terminal Alkynes H—C – C: + R X S N 2 H—C C—R + : X– The alkylating agent is an alkyl halide, and the reaction is nucleophilic substitution. The nucleophile is sodium acetylide or the sodium salt of a terminal (monosubstituted) alkyne.

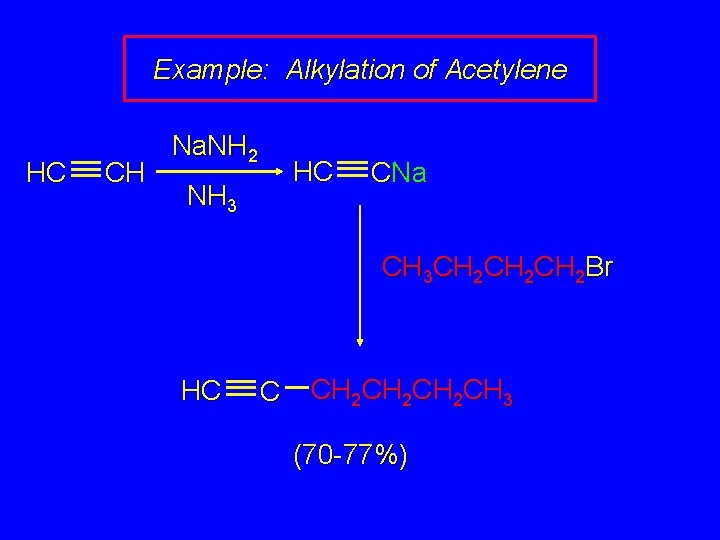
Example: Alkylation of Acetylene HC CH Na. NH 2 HC NH 3 CNa CH 3 CH 2 CH 2 Br HC C CH 2 CH 2 CH 3 (70 -77%)

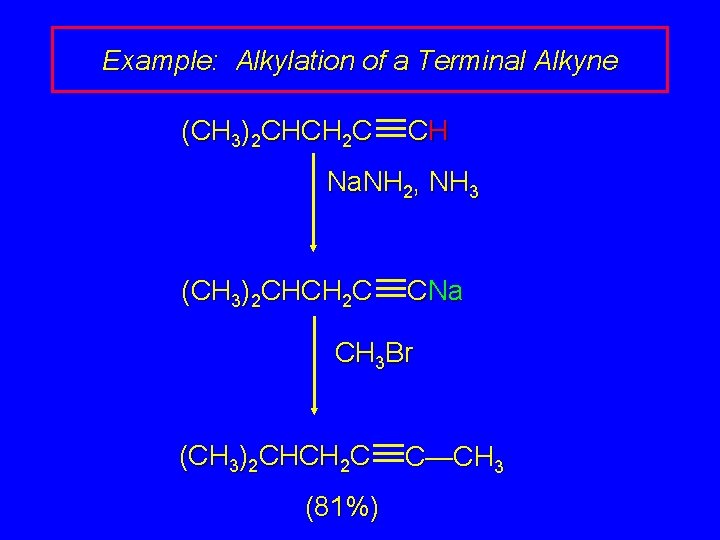
Example: Alkylation of a Terminal Alkyne (CH 3)2 CHCH 2 C CH Na. NH 2, NH 3 (CH 3)2 CHCH 2 C CNa CH 3 Br (CH 3)2 CHCH 2 C (81%) C—CH 3

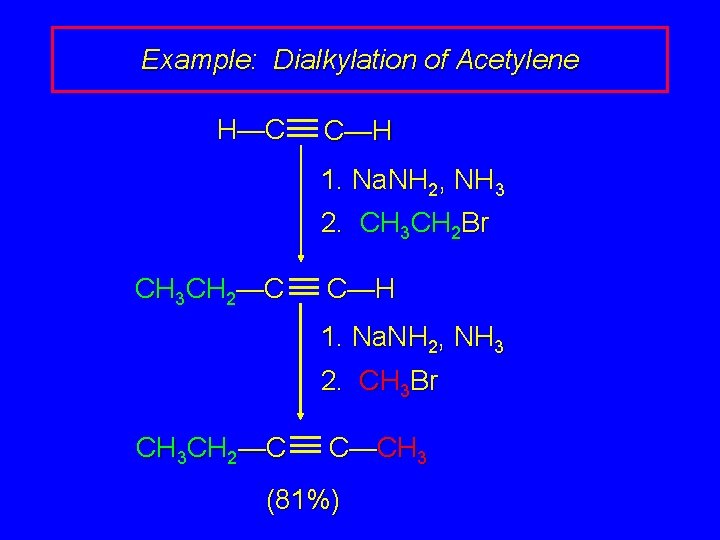
Example: Dialkylation of Acetylene H—C C—H 1. Na. NH 2, NH 3 2. CH 3 CH 2 Br CH 3 CH 2—C C—H 1. Na. NH 2, NH 3 2. CH 3 Br CH 3 CH 2—C C—CH 3 (81%)

Limitation Effective only with primary alkyl halides Secondary and tertiary alkyl halides undergo elimination

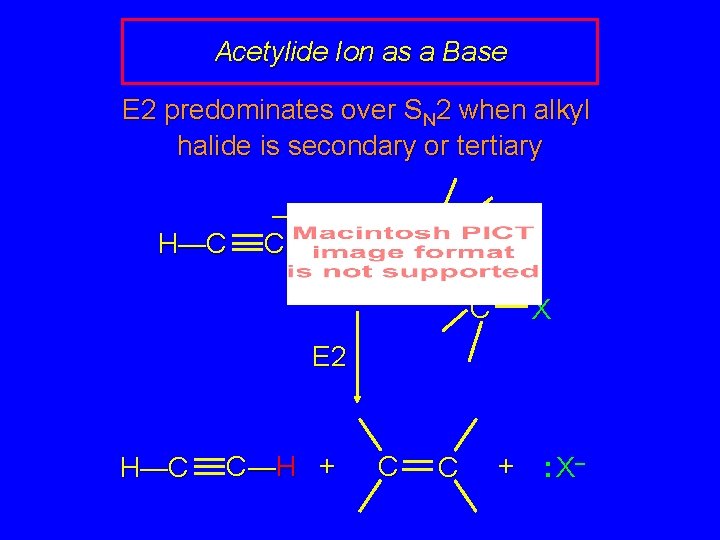
Acetylide Ion as a Base E 2 predominates over SN 2 when alkyl halide is secondary or tertiary H—C – C: H C C X E 2 H—C C —H + C C + : X–

9. 7 Preparation of Alkynes by Elimination Reactions

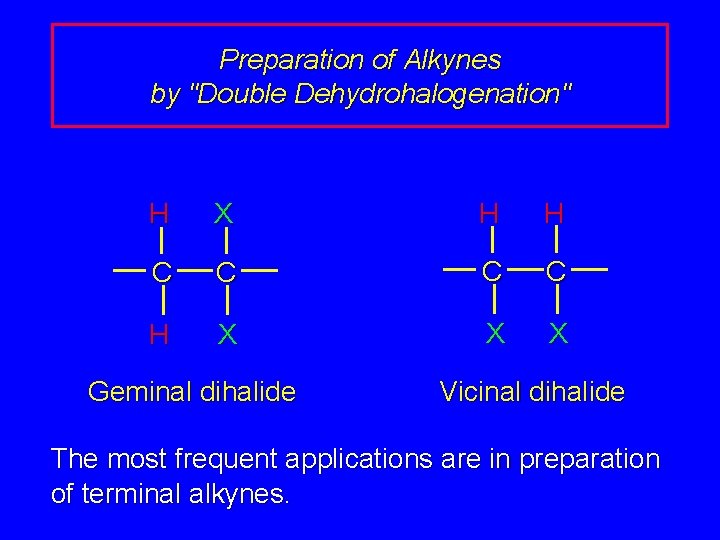
Preparation of Alkynes by "Double Dehydrohalogenation" H X H H C C H X X X Geminal dihalide Vicinal dihalide The most frequent applications are in preparation of terminal alkynes.

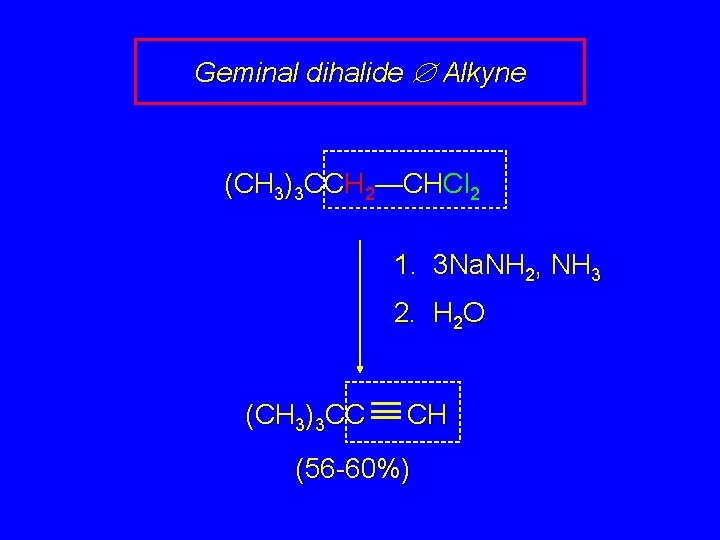
Geminal dihalide Æ Alkyne (CH 3)3 CCH 2—CHCl 2 1. 3 Na. NH 2, NH 3 2. H 2 O (CH 3)3 CC CH (56 -60%)

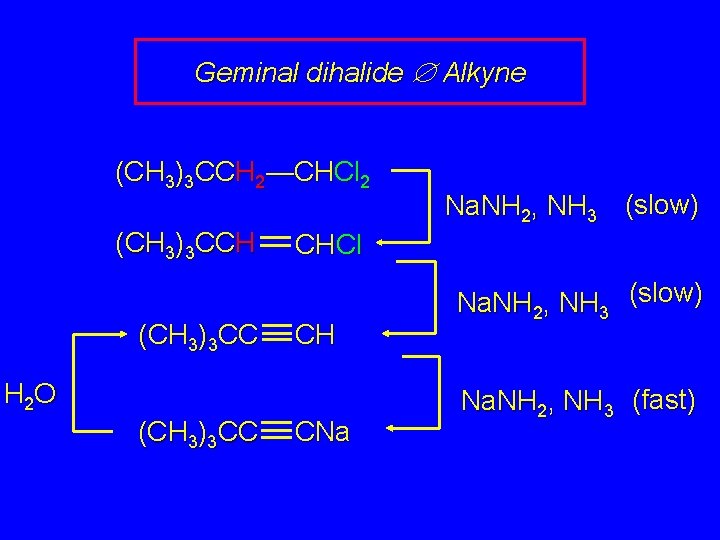
Geminal dihalide Æ Alkyne (CH 3)3 CCH 2—CHCl 2 (CH 3)3 CCH (CH 3)3 CC CHCl CH H 2 O (CH 3)3 CC Na. NH 2, NH 3 (slow) CNa Na. NH 2, NH 3 (slow) Na. NH 2, NH 3 (fast)

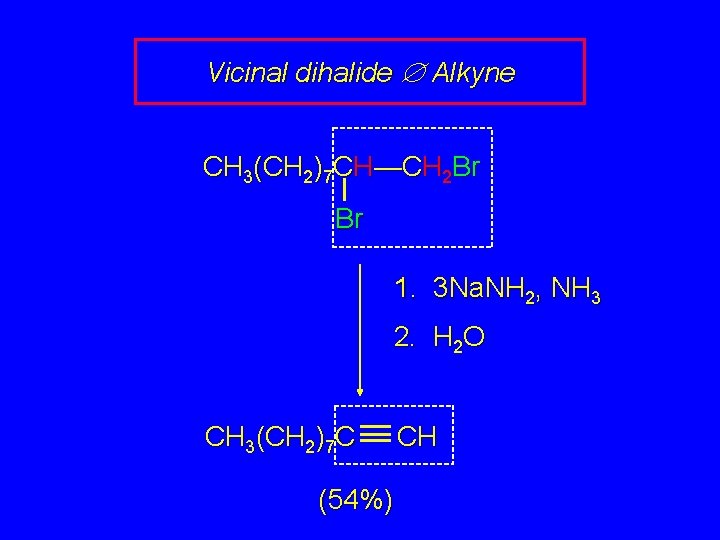
Vicinal dihalide Æ Alkyne CH 3(CH 2)7 CH—CH 2 Br Br 1. 3 Na. NH 2, NH 3 2. H 2 O CH 3(CH 2)7 C (54%) CH