9 1 Net Ionic Equations and Qualitative Analysis







- Slides: 21

9. 1 Net Ionic Equations and Qualitative Analysis Learning Goals … … write an ionic and net ionic equation … use qualitative analysis (flame test, solution colour and solubility) to identify ions in a solution

TYPES OF EQUATIONS i. Balanced chemical equation • chemical formulas used reactants and products • include states • balance all elements with coefficients ii. Total ionic equation • all soluble (aqueous) ionic compounds are written in their dissociated form (as ions) • include states and ionic charges iii. Net ionic equation • includes only what actually reacts • ions that appear on both sides of the equation are been removed and classified as spectator ions

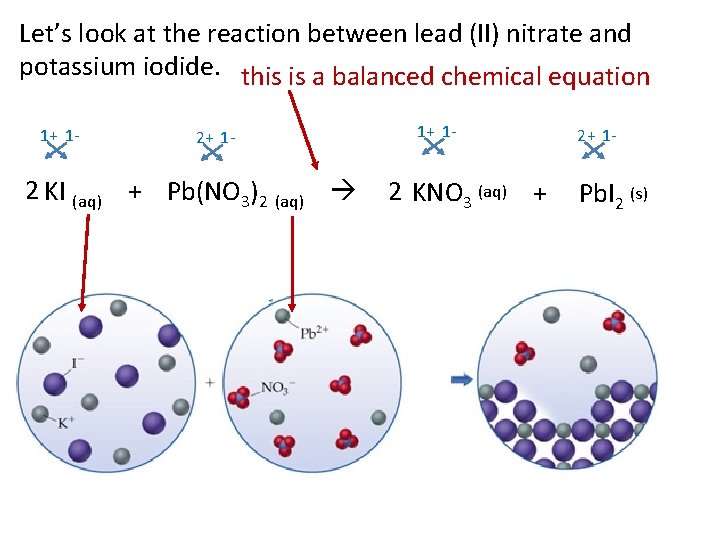
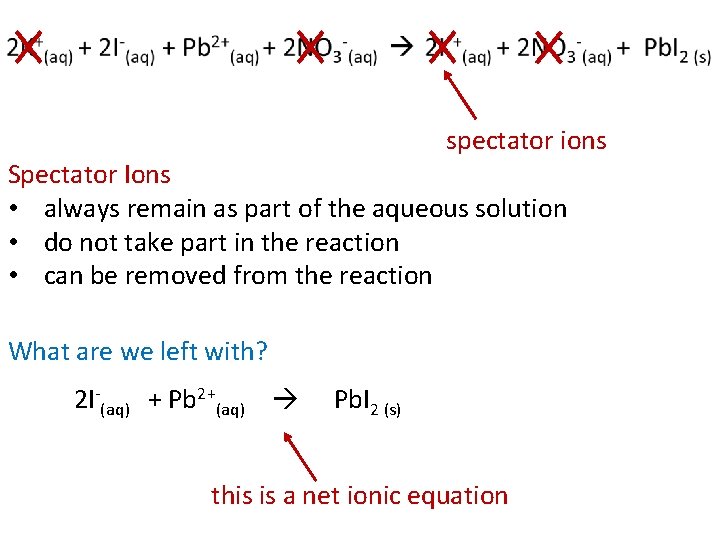
Let’s look at the reaction between lead (II) nitrate and potassium iodide. this is a balanced chemical equation 1+ 1 - 2 KI (aq) + Pb(NO 3)2 (aq) 1+ 1 - 2 KNO 3 (aq) + 2+ 1 - Pb. I 2 (s)


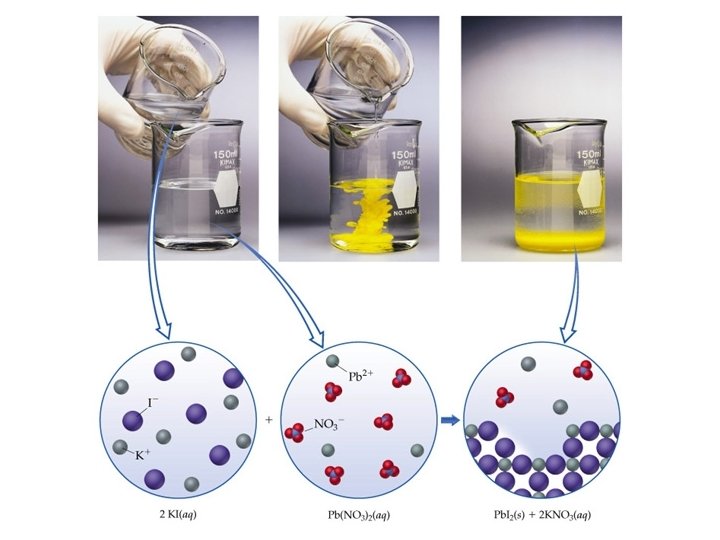
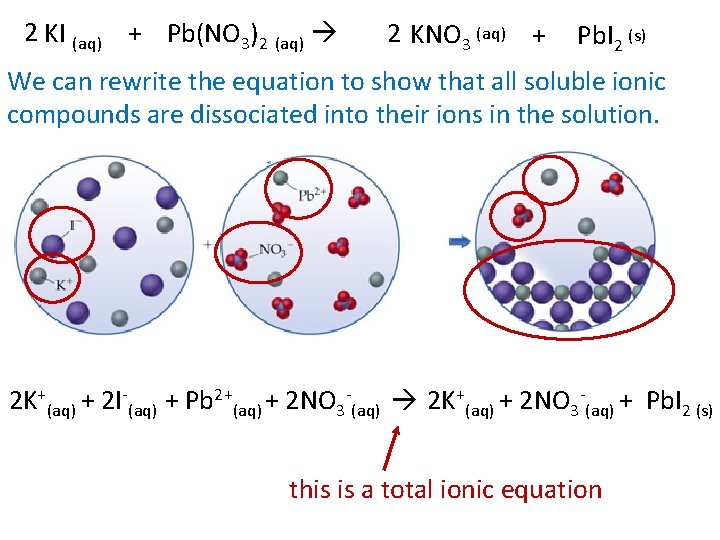
2 KI (aq) + Pb(NO 3)2 (aq) 2 KNO 3 (aq) + Pb. I 2 (s) We can rewrite the equation to show that all soluble ionic compounds are dissociated into their ions in the solution. 2 K+(aq) + 2 I-(aq) + Pb 2+(aq) + 2 NO 3 -(aq) 2 K+(aq) + 2 NO 3 -(aq) + Pb. I 2 (s) this is a total ionic equation

spectator ions Spectator Ions • always remain as part of the aqueous solution • do not take part in the reaction • can be removed from the reaction What are we left with? 2 I-(aq) + Pb 2+(aq) Pb. I 2 (s) this is a net ionic equation

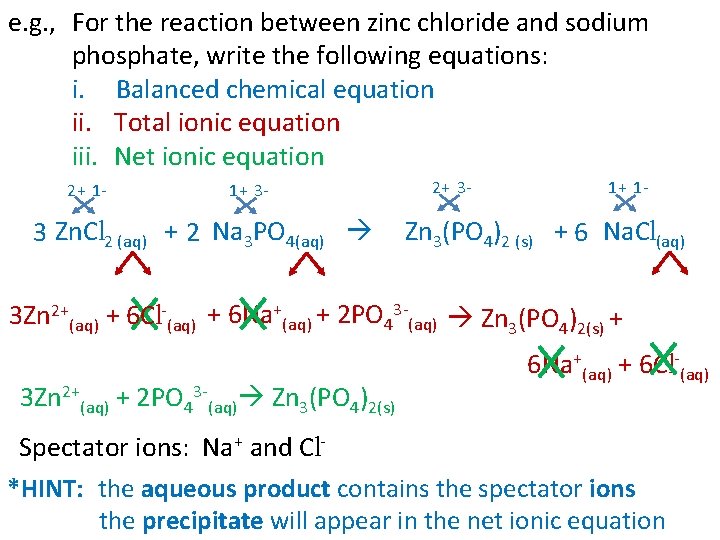
e. g. , For the reaction between zinc chloride and sodium phosphate, write the following equations: i. Balanced chemical equation ii. Total ionic equation iii. Net ionic equation 2+ 1 - 1+ 3 - 3 Zn. Cl 2 (aq) + 2 Na 3 PO 4(aq) 2+ 3 - 1+ 1 - Zn 3(PO 4)2 (s) + 6 Na. Cl(aq) 3 Zn 2+(aq) + 6 Cl-(aq) + 6 Na+(aq) + 2 PO 43 -(aq) Zn 3(PO 4)2(s) + 3 Zn 2+(aq) + 2 PO 43 -(aq) Zn 3(PO 4)2(s) 6 Na+(aq) + 6 Cl-(aq) Spectator ions: Na+ and Cl*HINT: the aqueous product contains the spectator ions the precipitate will appear in the net ionic equation

Qualitative Analysis • Qualitative Analysis identifies a substance in a sample by observation of physical and chemical properties. • You can often identify whether a cation is in a sample by observing one or more of the following: • Flame Test results • solution colour • precipitates formed with select aqueous solutions. • Qualitative analysis can tell you what ions are present in a solution.

1. Flame Test • many metals burn with a coloured light • flame colour can be used to identify the metal ion in the sample + 2+ +

1. Flame Test • many metals burn with a coloured light • flame colour can be used to identify the metal ion in the sample Flame Colours of Some Metals

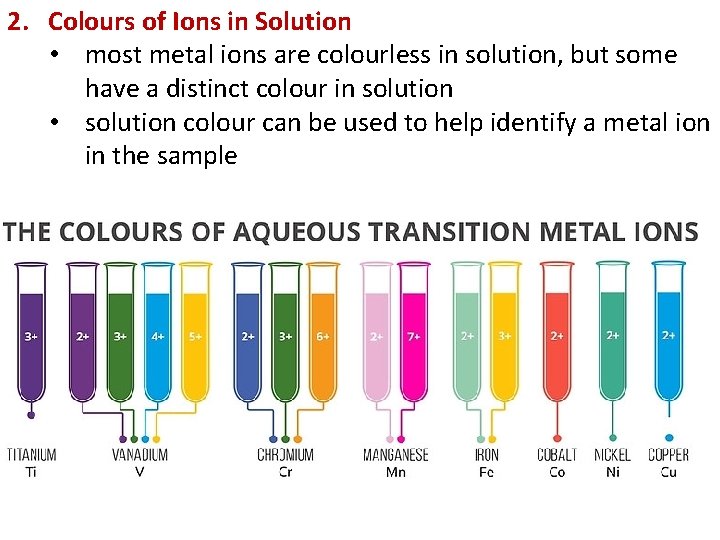
2. Colours of Ions in Solution • most metal ions are colourless in solution, but some have a distinct colour in solution • solution colour can be used to help identify a metal ion in the sample

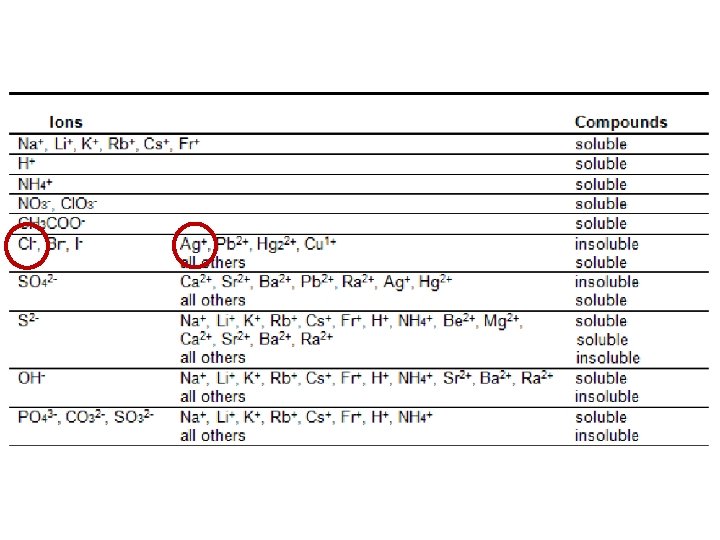
3. Precipitate Formation • • uses the solubility properties of ions to identify the presence of an unknown ion we can add a known reactant to a solution and observe whether a precipitate forms e. g. , How can you test for the presence of chloride ions in a sample of sea water? Let’s look at our Solubility Table …


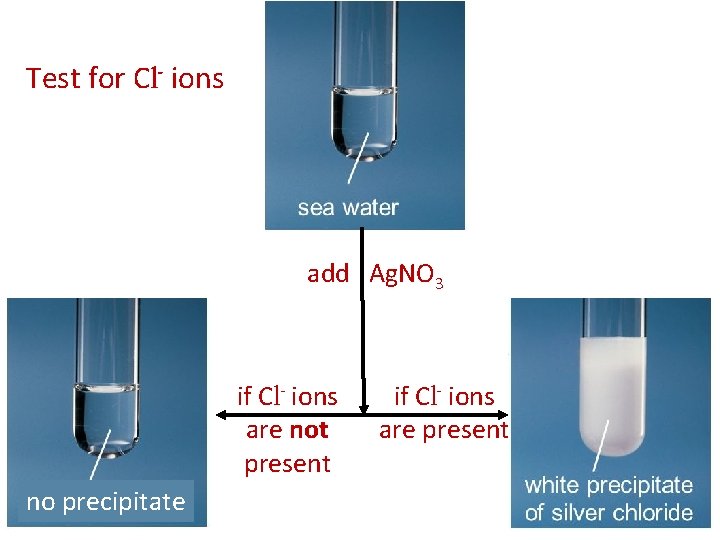
3. Precipitate Formation • • uses the solubility properties of ions to identify the presence of an unknown ion we can add a known reactant to a solution and observe whether a precipitate forms e. g. , How can you test for the presence of chloride ions in a sample of sea water? Let’s look at our Solubility Table … Cl- ions can be precipitated with Ag+ ions Ag+ could be obtained from a solution of Ag. NO 3 (we would need a non-reactive anion)

Test for Cl- ions add Ag. NO 3 if Cl- ions are not present no precipitate if Cl- ions are present

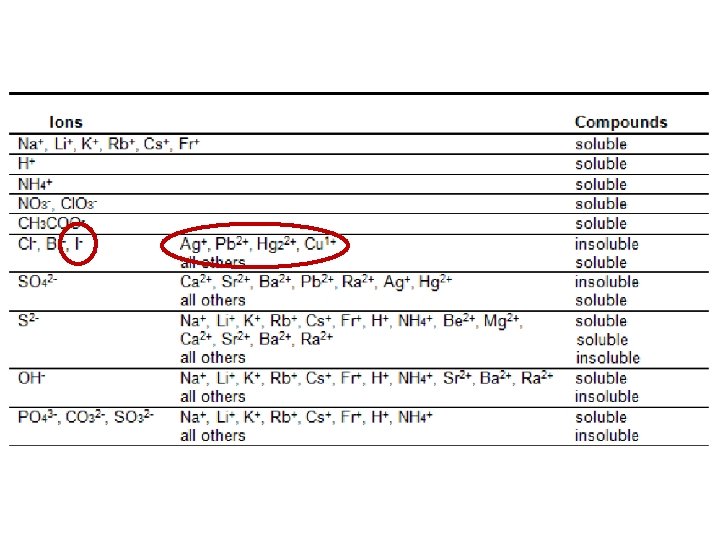
e. g. , An ion in solution forms a precipitate when sodium iodide, Na. I (aq), is added to the solution. The precipitate produces a blue-white colour when it is heated in a flame. What metal ion might be present in the solution? According to the Solubility Rules,


e. g. , An ion in solution forms a precipitate when sodium iodide, Na. I (aq), is added to the solution. The precipitate produces a blue-white colour when it is heated in a flame. What metal ion might be present in the solution? According to the Solubility Rules, • iodide will precipitate if the solution contains Pb 2+, Ag+, Cu+ and Hg 22+ • the ion must be one of these four According to the flame test results,

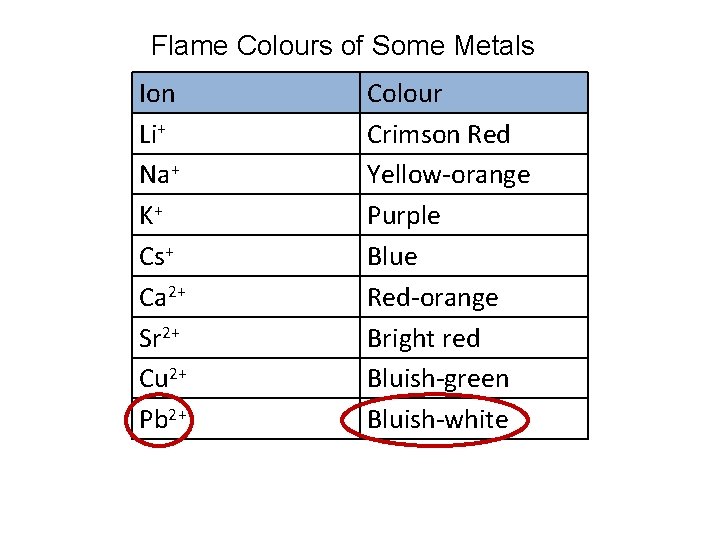
Flame Colours of Some Metals Ion Li+ Na+ K+ Cs+ Ca 2+ Sr 2+ Cu 2+ Pb 2+ Colour Crimson Red Yellow-orange Purple Blue Red-orange Bright red Bluish-green Bluish-white

e. g. , An ion in solution forms a precipitate when sodium iodide, Na. I (aq), is added to the solution. The precipitate produces a blue-white colour when it is heated in a flame. What metal ion might be present in the solution? According to the Solubility Rules, • iodide will precipitate if the solution contains Pb 2+, Ag+, Cu+ and Hg 22+ • the ion must be one of these four According to the flame test results, • Pb 2+ has a blue-white flame the metal ion might be Pb 2+

CAN I … … write an ionic and net ionic equation? … use qualitative analysis (flame test and solubility) to identify ions in a solution? HOMEWORK WS Qual. Analysis Problems p 410 #5 -10 p 414 #10, 12 -14